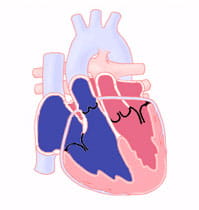
What is Transposition of the Great Arteries?
The "great arteries" in this defect refer to the aorta and the pulmonary artery. These are the two major arteries carrying blood away from the heart.
In cases of transposition of the great arteries, these vessels begin from the wrong ventricle. They are "transposed" from their normal position. The aorta starts from the right ventricle and the pulmonary artery from the left ventricle.
Other heart defects may occur along with transposition of the great arteries. About 25 percent of children with transposition will also have a ventricular septal defect (VSD). In a third, the branching pattern of the coronary arteries are unusual. Infants may also have narrowing below the pulmonary valve. This blocks blood flow from the left ventricle to the lungs.
Transposition creates a situation where the systemic (to the body) and pulmonary (to the lungs) circulations are working side by side and not together. This means the oxygen-poor ("blue") blood returning from the body and coursing through the right atrium and right ventricle is pumped out to the aorta and to the body. The oxygen-rich ("red") blood returning from the lungs and going through the left atrium and ventricle is sent back to the lungs by the pulmonary artery.
Unless there is some place in the circulation where the oxygen-rich and oxygen-poor blood can mix, the organs of the body will not get the oxygen they need.
To survive prior before surgery, the blood must mix somewhere in the heart. If present, a ventricular septal defect (VSD) will mixing. This does not allow enough mixing. Other places that mixing may occur are through an atrial septal defect (ASD) or a patent ductus arteriosus (PDA).
Signs and Symptoms of Transposition
Transposition of the great arteries can be diagnosed by a fetal ultrasound. This can be missed on a routine fetal ultrasound. Sometimes, a fetal echocardiogram done by specialists is needed to make a diagnosis of transposition in a fetus.
After birth, transposition is diagnosed in the first hours or days of life due to cyanosis or low oxygen levels. All babies have a patent ductus arteriosus (PDA) at birth that may allow enough mixing to prevent severe cyanosis. As the ductus arteriosus closes, as it will in the first hours or days of life, cyanosis gets more severe.
Rapid breathing is seen. This is due to the low oxygen levels in the blood. The infants do look uncomfortable breathing this fast.
A heart murmur heart murmur is often absent in the first days or weeks of life. If there is a site where blood mixing allows for safe oxygen levels, children will often develop signs and symptoms of congestive heart failure over the course of the first weeks or months of life.
Untreated, over 50 percent of infants with transposition will die in the first month of life. Ninety percent will die in the first year.
Diagnosing Transposition of the Great Arteries
When a newborn with significant cyanosis is first seen, they are often placed on supplemental oxygen. In cases of lung disease this often will improve the oxygen levels. With cardiac problems such as transposition there will be little effect on the child's oxygen levels. Failure of this "hyperoxia test" is often the first clue that a baby has a cardiac defect.
Echocardiography can quickly show the abnormal connections of the great arteries and other features of the cardiac anatomy. This includes the presence and size of an atrial or ventricular septal defect and the branching patterns of the coronary arteries.
If questions about the anatomy, such as the coronary artery pattern remain, cardiac catheterization or cardiac MRI may be done to get more details of the defect.
Treatment
The immediate management of an infant with transposition focuses on getting safe oxygen levels. Stable cardiac and pulmonary function is important.
A continuous infusion of prostaglandin, a medication that will keep the ductus arteriosus open, is usually started when the diagnosis is suspected or confirmed. This will allow some mixing of oxygen-rich blood with oxygen-poor blood.
A procedure called a "balloon atrial septostomy" is often done once the diagnosis is confirmed. Before birth, all babies have a connection between the right atrium and the left atrium (called a foramen ovale). After birth, this normal connection may allow some mixing of blood. It may not provide enough mixing. The foramen ovale may be made bigger or stretched with a balloon, which will improve mixing. This balloon atrial septostomy procedure is done by passing a special balloon-tipped catheter into the heart from either a vessel in the umbilicus or a vessel in the groin. Often, the procedure is done at the bedside, with guidance from an echocardiogram. Occasionally, the procedure will be done in the catheterization laboratory.
A large atrial septal defect is created and allows excellent mixing of oxygen-rich and oxygen-poor blood. The body's oxygen saturation will stay in a safe range. After this procedure, the ductus arteriosus is no longer necessary. The prostaglandin infusion can be discontinued.
Babies can be stabilized for the short term. Surgical correction of the defect is always needed. In most cases, corrective surgery is done in the first week of life. In more complex cases, such as those with narrowing below the pulmonary valve (pulmonary stenosis), the time for surgery can vary.
In most cases of transposition, an arterial switch surgery is done. The arterial switch surgery involves cutting off the aorta and pulmonary arteries just above the point where they leave the heart. Part of this surgery is reconnecting them to the proper ventricle. The valve stays attached to the ventricle, so what was once the pulmonary valve is now the aortic valve. The aortic valve becomes the pulmonary valve.
Watch Video: Arterial Switch Operation for Transposition of the Great Arteries
Results of Treatment
Since the arterial switch surgery reconstructs the heart to a normal situation, long-term cardiac function should be excellent.
In a small percentage of children, narrowing (stenosis) may occur at the sites where the aorta and pulmonary artery are reattached. The narrowing may occur months or years following surgery. It may need additional treatment. Options for treating this narrowing include cardiac catheterization with balloon dilation of the narrowed area or another surgery.
Sometimes, the aorta becomes larger in its portion immediately after it arises from the left ventricle.
Rarely, there may be problems with flow through the coronary arteries.
Patients (after arterial switch surgery) usually have normal ventricular function and no heart rhythm abnormalities.
In later years, patients with the arterial switch surgery have good quality of life. They can do physical activity and sports without any difficulty or restrictions. Even though patients are expected to do well after the arterial switch surgery, long-term follow-up with the cardiologist is important.
Adult and Adolescent Management
All patients with transposition of the great arteries need to be followed by a congenital heart expert for life.
Learn more about the Adolescent and Adult Congenital Heart Disease Program.