What is an Interrupted Aortic Arch?
The aorta is the main blood vessel that carries oxygen-rich blood away from the heart to the organs of the body. After it leaves the heart, it first goes into the chest to give off blood vessels to the arms and head. Then, it turns downward. This path forms a semicircular arch. This leads toward the lower half of the body
Interrupted aortic arch (IAA) means a missing portion of the aortic arch.
There are three types of interrupted aortic arch. They are grouped according to the area of the missing piece
- Type A: The interruption occurs just past the left subclavian artery. About 30 percent to 40 percent of the infants with interrupted aortic arch have type A.
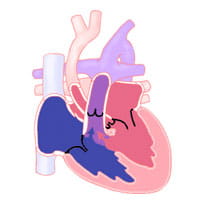
- Type B (diagram): The interruption occurs between the left carotid artery and the left subclavian artery. Type B is the most common form of interrupted aortic arch. It accounts for about 53 percent of reported cases.
- Type C: The interruption occurs between the innominate artery (an artery that comes from the aortic arch, towards the right side of the body) and the left carotid artery. Type C is the least common form of interrupted aortic arch. This type counts for about four percent of reported cases.
Interrupted aortic arch is thought to be a result of flawed development of the aortic arch system during the fifth to seventh week of fetal development. This defect is usually associated with a large ventricular septal defect (VSD). Patients with interrupted aortic arch (particularly those with type B) often have a genetic disorder called DiGeorge syndrome. In addition to interrupted aortic arch, patients with DiGeorge syndrome may have problems with low calcium, developmental delay and immune system abnormalities.
Associated Problems
In patients with interrupted aortic arch, oxygen-rich blood from the left side of the heart is not able to reach all areas of the body. This is the case because of the defect in the aortic arch. An infant with interrupted aortic arch must depend on an another way to get blood flow to the lower body.
Normally, a fetus has an extra arterial connection called a ductus arteriosus. The ductus arteriosus is critical to survival in the womb. Shortly after birth, the ductus arteriosus usually closes. If the ductus arteriosus stays open, it is called a patent ductus arteriosus (PDA).
In a child with interrupted aortic arch, the PDA gives another way to get enough blood flow to the lower body.
While the ductus arteriosus is open, infants may not have noticeable symptoms. They may not be diagnosed. As the ductus arteriosus starts to close, the infant begins to show signs and symptoms of not having enough blood flow to the area. Not getting enough blood flow to the body may lead to severe symptoms. These may include shock and congestive heart failure.
If a ventricular septal defect is present, blood will be moved (shunted) from the left side to the right side of the heart. This shunting causes an increase blood flow to the lungs. This leads to congestive heart failure as well.
Signs and Symptoms
Signs and symptoms of poor perfusion or congestive heart failure may develop when the ductus arteriosus begins to close. This is around the first day or two of life.
The infant may develop weakness, tiredness, poor feeding, rapid breathing, fast heart rate, or low oxygen levels. The low oxygen levels may show more when measured in the legs and feet.
This condition can worsen and lead to shock. The infant will be pale, blotchy and cool. The infant will likely have fewer wet diapers and poor pulses. Poor pulses are seen more in the lower extremities.
Diagnosis
Diagnosis of interrupted aortic arch may be suspected based on the symptoms the infant has. It is confirmed by an echocardiogram. Once the diagnosis is confirmed, treatment and surgical intervention are important.
Treatment
Immediate treatment includes a prostaglandin infusion. Prostaglandin is a medicine that is given intravenously (IV). It keeps the ductus arteriosus open. This allows blood flow to the lower body until surgery is done to fix the interruption in the aortic arch.
Goals of treatment to stabilize and support the infant until surgery happens. Treatment may include:
- Intubation (endotracheal or “breathing tube” placed in the airway)
- Diuretic therapy (water pills) to help the infant pee out the extra fluid
- Giving inotropic medications (to help improve the pumping action of the heart)
- Monitoring and correcting abnormal blood gases (carbon dioxide and oxygen levels in the blood) and electrolytes (potassium and calcium levels in the blood)
- Nutrition support
The goal of surgery is to reconnect the aortic arch to create a continuous "tube" and close the ventricular septal defect. Surgery is usually done quickly after the infant is stabilized (usually in the first few days of life).
Open-heart surgery will be done to connect the two separate portions of the aorta and close the ventricular septal defect. The PDA will also be tied off (ligated).
Complications after interrupted aortic arch repair may include continued obstruction. Stenosis (narrowing) at the aortic repair site may also happen.
The aortic valve or the area below the valve are often small and may not grow. This can result in stenosis (narrowing) months or years after surgery.
Surgery
Risk is higher if the child has a small size of the aortic valve region. The surgery risk is higher is the child is ill or unstable before surgery.
Survival is not possible without surgery. Survival after complete repair of the aortic arch and ventricular septal defect in the newborn period is 90 percent.
Survival is not possible without surgery. By comparison, survival after complete repair of the aortic arch and ventricular septal defect in the newborn period is 90 percent or greater in most pediatric heart centers.
Long-term follow-up by the cardiologist to watch growth of the aortic valve region and the reconstructed aortic arch is important. Another surgery to address further problems with these areas may be needed in 10 to 20 percent of patients.
Adult and Adolescent Management
An interrupted aortic arch is a rare congenital defect. It has always been repaired in childhood. Any adult patient with a history of aortic arch stenosis will need lifelong care by an expert in congenital heart defects. Problems may occur at the site of the original surgical repair. It may be related to other congenital heart defects that the patient was born with.
A ventricular septal defect (VSD) is a very common congenital anomaly. This means that there is a hole in the part of the heart that separates the two main pumping chambers. This allows blood to flow between the heart chambers. If the hole is small, blood flows from the left heart through the right heart to the lungs. But the amount is small enough that the hole does not need to be closed. Patients with such small VSDs have two major risks. The first is of infective endocarditis. This is an infection of the heart that can be fatal if not recognized and treated. The second is leakage of the aortic valve. This may eventually need surgical repair or replacement.
Patients with larger VSDs usually have had repair as children. Some of them will have leaks in which there is a small amount of blood flow across the ventricular septum. The major risk is endocarditis. If the patient had a large VSD and has not been repaired by adult life, they will usually have high blood pressure in the lungs (pulmonary arterial hypertension). This can cause Eisenmenger syndrome. This is a condition in which the blood pressure in the lung becomes so high that blue blood starts to mix with red blood. This causes the patient to develop a bluish tinge to the skin and other features.
Learn more about the Adolescent and Adult Congenital Heart Disease Program.