A Big Idea Grows From Helping Tiny Babies
Start-up company pursuing a dramatic improvement to artificial surfactant reflects the future of pediatric drug development
Fueled by more than $150 million a year in research funding, innovation happens every day at Cincinnati Children’s. But even the best ideas cannot improve child health until they manage to cross a funding desert that stands between intriguing concepts and real-world therapeutics.
 |
|
"Many of the babies we treat, especially the very small babies, have good initial responses to surfactant replacement, which saves their lives." |
“What you have is a no-man’s land between what academic medical centers will typically do and what the big drug companies are looking for,” says Steven Linberg, President and CEO of a Cincinnati Children’s spin-off company formed in 2011, Airway Therapeutics. “Universities generally are not in the drug development business and drug companies don’t want to sink a lot of money into development when they aren’t sure of the returns.”
Start-ups like Airway Therapeutics fill that gap, Linberg says. The company’s key initiative will be recombinant human surfactant protein D (SP-D), a product based on years of research led by Jeff Whitsett, MD. Whitsett, Co-Director of the Perinatal Institute at Cincinnati Children’s, helped develop the first generation of artificial surfactants, which enable premature babies to breathe more easily until their lungs are fully developed.
More recently, Whitsett’s work has focused on making surfactant therapy more effective. He and his colleagues found that naturally occurring surfactant protein D— which is not currently included in the available surfactants used for preemies — may play a crucial role in preventing later respiratory problems.
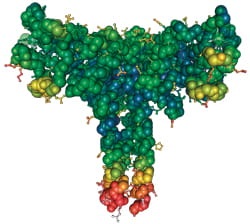
Surfactant Protein D |
|
This naturally-occurring substance is not currently used in treatments for premature infants. But it could prevent bronchopulmonary dysplasia and other respiratory problems later in life. |
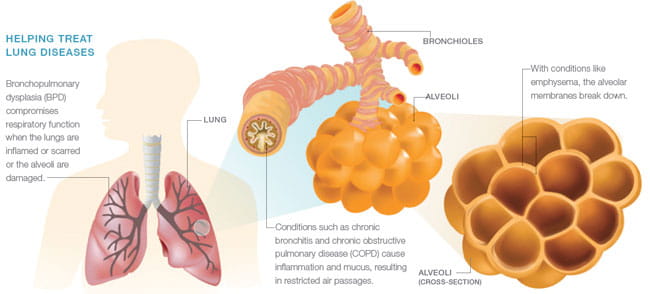
“Many of the babies we treat, especially the very small babies, have good initial responses to surfactant replacement, which saves their lives,” Whitsett says. “But many develop bronchopulmonary dysplasia. These babies require long hospitalizations with oxygen support and they have recurrent viral and bacterial infections that go on for many months.”
Airway Therapeutics believes that adding surfactant protein D back into a marketed surfactant where nature originally put it could dramatically reduce intensive care stays and deaths among these infants.
Use Beyond Infants
Surfactant protein D also may prove useful in treating other conditions, including cystic fibrosis and smoking-related chronic obstructive pulmonary disease (COPD), Whitsett says.
“We know from animal studies that SP-D is dramatically protective against septic shock in newborns. We also know that smoking destroys SP-D, making emphysema more likely to develop. So it turns out that SP-D has implications throughout life,” he says.
Cincinnati Children’s has been making a human recombinant form of SP-D for several years to use in lab tests. Airway Therapeutics is leading the effort to scale up production for use in human clinical trials.
Currently, the protein is going through validation tests to confirm that the production process meets the strict quality standards required for human studies, Linberg says. If successful, a Phase III human clinical trial could begin in 2014.
 |
A Model For The Future
The surfactant D project reflects the kind of translational research to be expanded at the new Clinical Sciences Building, a 15-story research tower under construction at Cincinnati Children’s main campus.
The building, along with other expanded capabilities at Cincinnati Children’s, helps address a challenging reality for pediatric medicine. With large pharmaceutical companies often unwilling to take risks to develop drugs with small market potential, pediatric medical centers must take on more of the early-stage work themselves.
Big Ideas Begin At Cincinnati Children’sResearch at Cincinnati Children’s has led to the creation of seven start-up companies in recent years:
|
“I see this as the future for drug development,” Linberg says. “The days of big pharmaceutical companies developing all their products in-house are over.”
Cincinnati Children’s is becoming a leader in this trend. Not only has the medical center supported the formation of Airway Therapeutics, it invested funds to launch the company.
“What drug companies are looking for is a little bit more development, a little more proof of concept, to give them a better idea of what they’re gambling on,” Linberg says. “Cincinnati Children’s has been very helpful. They did much more than sign a licensing agreement. They put up money and they’ve remained involved scientifically.”
When and if the improved version surfactant reaches market, Cincinnati Children’s stands to benefit from milestone payments and royalties that will help support more research.
The medical center already has launched seven start-up companies and there will likely be more. Scientists at Cincinnati Children’s filed a record 188 invention disclosures in the past year, according to the medical center’s Center for Technology Commercialization (CTC). That’s up from a five-year average of 97 disclosures a year.