Current Projects
Stress in the development and progression of epilepsy
Stress is widely recognized as a factor for provoking seizures in many patients with epilepsy. But despite clear evidence for a link between stress and seizures in humans, exactly how stress affects the occurrence of seizures in patients with epilepsy is not well understood. A better understanding of this process could lead to new strategies to control seizures. The goal of this project, therefore, is to explore the relationship between stress, stress hormones and seizures in animal models of epilepsy.
2008-2010 − Charles L. Shor Foundation for Epilepsy Research. Cincinnati.
SC Danzer, coprincipal investigator (in collaboration with James Herman, UC Department of Psychiatry).
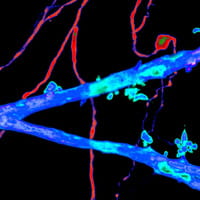
Short- and long-term impact of neonatal seizures on hippocampal granule cell integration
Seizures in children are common. The long-term consequences of these seizures, however, are still unclear. We examine the effect of neonatal seizures on the development of a brain region critical for learning, language development, cognition and imagination. These studies will help us determine how neonatal seizures may harm young brains and will guide the development of treatments to mitigate that damage.
2009-2011 − National Institutes of Health, 1R03-NS-064378-01.
SC Danzer, principal investigator
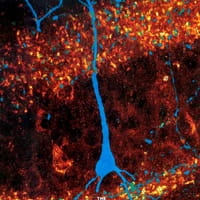
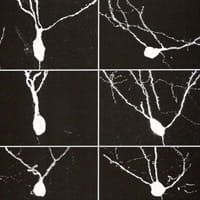
Selective disruption of hippocampal dentate granule cells in autism: impact of PTEN deletion
This research intends to determine whether selective disruption of late-generated neurons – specifically hippocampal dentate granule cells – contributes to the development of autism. Although the proximal cause of autism is undetermined in most cases, the late generation of these neurons (in the late embryonic period and in infancy) may make them uniquely vulnerable to a variety of insults faced by the newborn child (e.g., infection). A connection between seizure-generated disruption in these cells and the development of autism could offer a path to mitigate or prevent autism. We will examine the role these neurons play by selectively eliminating genes implicated in autism in these cells in transgenic mice.
2009-2014 − National Institutes of Health, 1R01-NS-065020-01.
SC Danzer, principal investigator.
Contributions of aberrant granule cell integration to the development of epilepsy
In adults, new brain cells are born daily in the hippocampus, a region that plays a role in common forms of epilepsy. Abnormal incorporation of these new cells into the brain may promote the development of the disease. We will investigate the role these new neurons play in the pathology, development and maintenance of epilepsy. By determining how normal brains become epileptic, we can begin to develop therapies to delay, halt and ultimately reverse the process.
2009-2014 − National Institutes of Health, 1R01-NS-062806.
SC Danzer, principal investigator.