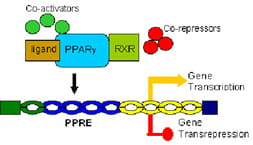
The Kaplan Laboratory is conducting a Phase 1 clinical trial to evaluate the pharmacokinetic characteristics, safety profile, and effect on inflammatory markers of pioglitazone in patients with severe sepsis and septic shock. Pioglitazone belongs to a class of drugs called thiazolidinediones (TZDs), which are commonly used in patients with type 2 diabetes. The preclinical work was initiated by
Basilia Zingarelli, MD, PhD and in collaboration with Dr. Zingarelli the Kaplan Laboratory continues to investigate the critical molecular mechanisms involved in the anti-inflammatory effects of TZDs in sepsis. The Pioglitazone in Pediatric Sepsis study is a collaborative effort with
Hector Wong, MD (director, Division of Critical Care Medicine),
Alexander Vinks, PharmD, PhD (director, Division of Clinical Pharmacology), the Translational Research Trials Office (TRTO), and has been funded by the Center for Clinical and Translational Science and Training (CCTST). This phase 1 trial is the first basic research effort in the Division of Critical Care Medicine at Cincinnati Children’s that has been directly translated to the bedside of critically ill children. We submitted an investigational new drug (IND) application from the FDA and are deemed safe to proceed. The trial is registered with ClinicalTrials.gov (
NCT01352182).