Research Interests
Research Interests
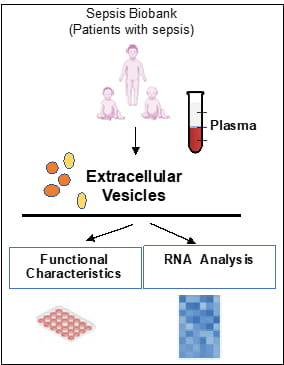
Extracellular vesicles are spherical microparticles (0.03–5 μm) which are released from almost any cell type into a variety of bodily fluids including plasma, serum, saliva, cerebrospinal fluid, breast milk, urine, and others. Depending on the cell of origin, these vesicles can contain many constituents of a cell, such as DNA, RNA, lipids, metabolites, and proteins. This cell-specific cargo can be selectively taken up by neighboring or distant cells, leading to reprogramming of the function of recipient cells. The laboratory is interested in understanding how extracellular vesicles can affect the function of immune competent cells, thus affecting the response to infections. Because circulating extracellular vesicles manifest characteristics of the cell of origin, they can be used as liquid biopsy for molecular diagnostics in patients with sepsis. The approach we are undertaking is to characterize the association of the RNA content, including small non-coding microRNAs, of plasma extracellular vesicles with severity of sepsis. To this aim, we also are establishing a reliable biorepository of extracellular vesicles which are isolated from plasma of critically ill patients and can be used for biomarker research.