Teaching an Old Dogma New Tricks
Research By: Katherine Yutzey, PhD
Post Date: June 30, 2019 | Publish Date: March 15, 2016
“The old dogma was you can’t get new heart muscle, and now we and some other groups in the field are finding that you can.”
—Katherine Yutzey, PhD
One seemingly impassable barrier on the path to one of medicine’s Holy Grails—regenerating heart muscle injured by heart attack or disease—has been the long-held belief that mature heart muscle cells do not regrow.
Based on studies in mice, cardiomyocytes undergo a few cycles of mitotic cell division after birth, end up with a double nucleus, then forever stop regenerating—or so the dogma goes.
But heart researchers at Cincinnati Children’s are trying to upend what has been one of the hard facts of life after cardiac infarction, or being born with a serious heart anomaly. The effort is producing some exciting, but still preliminary, results. This includes one finding that developed in such an unexpected manner that the scientists initially did not believe what they found.
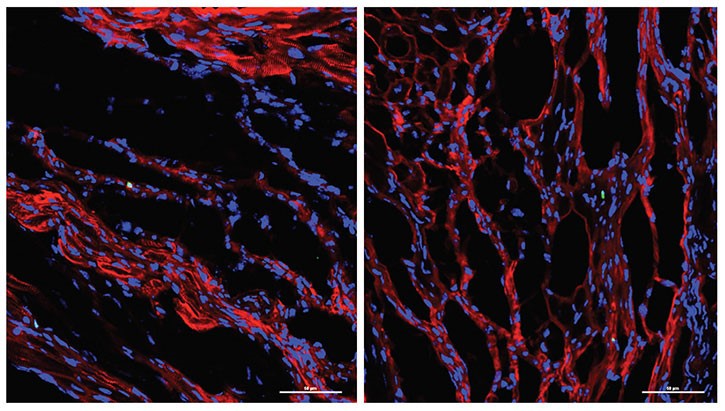
Led by Katherine Yutzey, PhD, Division of Molecular Cardiovascular Biology, and Fuli Xiang, PhD (a former member of Yutzey’s lab, now at Novartis), the research team found that over-expression of the transcription factor Tbx20 in the infarcted and damaged hearts of mice prompted the hearts to partially regenerate. Treated mice regained 60-75 percent of their heart function.
Transcription factors are proteins that tell other genes what do to. From a surprising laboratory observation by Santanu Chakraborty, PhD, (Yutzey’s post-doctoral fellow at the time) the scientists learned Tbx20 sets off a cascade that gets mature cardiac muscle cells to act more like fetal cardiomyocytes. Scientists know that fetal cardiomyocytes, which help spur initial heart development, are able to regenerate and proliferate.
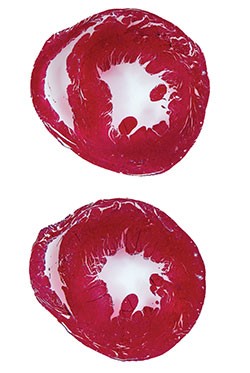
The potential implications of the team’s discovery were startling enough that it prompted investigators to check and recheck the data for the reality of the findings to set in.
“When the observation was first reported to me I didn’t believe it,” Yutzey says. “I’ve had three different postdocs work on this now, trying to show the same finding in different ways, and finally it became evident that it was working.
“The old dogma was you can’t get new heart muscle, and now we and some other groups in the field are finding that you can,” she says. “The question is how you make that work so it’s therapeutically beneficial to patients.”
High-risk, higher stakes
The research clearly falls into a high-risk category, which essentially means the chances of failure are considerable, according to Yutzey. Also considerable are the potentially high benefits if the research leads to useful therapies.
According to the U.S. Centers for Disease Control and Prevention, unhealed heart attack damage is the leading cause of heart failure, contributing to 287,000 deaths a year in the United States alone. Meanwhile, congenital heart defects affect about one percent—or about 40,000—births a year in the U.S. The estimated hospital costs for people with congenital heart disease exceeds $1.9 billion.
And while today’s advancing surgical techniques can reconfigure some malformed hearts to allow more children born with cardiac defects to survive into adulthood, those survivors often need complex, life-long follow-up care.
When age matters
One of the questions Yutzey and her colleagues want to answer is what age and health status would be best for performing complex corrective procedures in children. Should children be younger or older? Much could depend on the amount of time after birth that human cardiomyocytes start to lose their fetal characteristics and regenerative potential.
“Mice have this ability to regenerate heart muscle if they are injured for about a week after birth. We have no idea when it stops in humans, and obviously you can’t study this in human babies,” Yutzey explains.
“When the surgeons do these repairs it’s a pretty severe operation. They have to move things around and make connections that didn’t exist before. If there was a way to optimize the growth of new heart muscle after a repair like this, a huge basic question becomes finding out how long the human heart can heal itself after birth.”
Yutzey is part of a wide-ranging collaborative research project with Cincinnati Children’s Heart Institute, including cardiac surgeon Farhan Zafar, MD, and senior research assistant Scott Baker to try and find out. The planned work includes experimental surgical processes dovetailing with detailed laboratory analyses.
“The surgeons tell us that younger infants can do better after having these complex surgeries and there is more plasticity in their hearts and tissues, but none of us have any data on this,” she says.
A long journey ahead
The next phase of the team’s research will involve testing Tbx20 in preclinical models that offer closer comparisons to the human heart.
For its initial work, the team studied mice that were engineered to overexpress Tbx20. Going forward, researchers plan to employ a viral vector to deliver Tbx20 to injured heart tissue. If successful, this approach has more potential for clinical use.
These steps will take time, but at least now, a pathway to that Holy Grail of heart research appears to be open for exploring.
—By Nick Miller
(This article originally appeared in the Summer 2017 issue of Research Horizons)
| Original title: | Overexpression of Tbx20 in Adult Cardiomyocytes Promotes Proliferation and Improves Cardiac Function After Myocardial Infarction |
| Published in: | Circulation |
| Publish date: | March 15, 2016 |
Research By