Dose Escalation Sharply Improves Hydroxyurea Benefit for Children with Sickle Cell Anemia
|
Top Breakthrough Discovery | Published June 2020 in The New England Journal of Medicine |

Russell Ware, MD, PhD, Teresa Latham, MA, and Adam Lane, PhD
The clinical trial results in Uganda were so clear the study was stopped early.
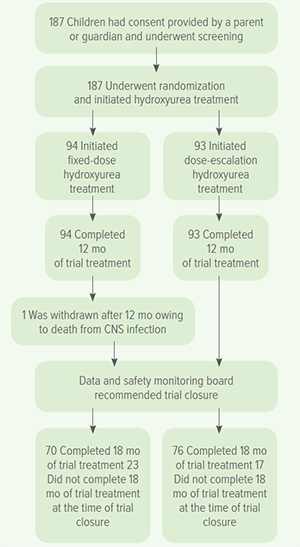
Rather than using a single, common dosage of the drug hydroxyurea, escalating the dose to a maximum tolerated level significantly lowered the complications from sickle cell anemia.
Gradually increasing dosages to about 50% higher than previously used levels reduced hospitalizations by 79%, transfusions by 70%, the risk of acute chest syndrome or pneumonia by 73%, and the risk of a vaso-occlusive pain crisis by 57%, according to data from the NOHARM MTD study. (Novel use Of Hydroxyurea in an African Region with Malaria – Maximum Tolerated Dose).
The clinical trial results were the latest step forward for a years-long effort led by Russell Ware, MD, PhD, at Cincinnati Children’s and collaborators to demonstrate that hydroxyurea offers an effective, low-cost treatment for sickle cell anemia without increasing malaria risks.
Collaborators on the NOHARM-MTD study included experts from Makerere University in Kampala, Uganda, and the Indiana University School of Medicine.
“Our study’s data safety and monitoring board noted a highly significant difference between the treatment groups, with the children on escalated dosing having superior clinical results but the same number of side effects, so at their recommendation we halted the trial and moved all of the children to that escalated dosing strategy,” said Robert Opoka, MMed, who oversaw the study at Makerere University.