Early-Life Heart Muscle and Nerve Development Depends Upon Special Set of Cells
Published August 2020 | PNAS
A research team at Cincinnati Children’s has shed new light on the mechanisms at work as mammalian heart muscle loses the ability to regenerate, a discovery that may someday help scientists find effective ways to prompt damaged hearts to heal themselves.
In a study led by first author Luis Hortells, PhD, and senior author Katherine Yutzey, PhD, the team found that postnatal cardiac fibroblasts include a transient population of highly proliferative cells carrying the gene Periostin (Postn+). This population is required for cardiac nerve development and cardiomyocyte maturation soon after birth.
However, these Postn+ cells largely vanish during the first month after birth. After that, heart development shifts from hyperplasic to hypertrophic growth, and nerve development patterns change. By adulthood, the mature heart becomes largely unable to regenerate new muscle cells to replace damaged ones.
Discovering a specialized population of cells within developing heart tissue was made possible through single-cell analysis techniques that helped the team tease out new details about gene expression and cell function, including new information about crosstalk between fibroblasts, neurons and glial cells.
In adult hearts, some Postn+ cells reactivate to form post-injury scar tissue. But during the postnatal period, Postn+ cells display higher levels of expression of Egf, Igf2, and neuron growth-related genes.
Co-authors from Cincinnati Children’s also included Iñigo Valiente-Alandi, Zachary Thomas, Emma Agnew, Dan Schnell, Allen York, Ronald Vagnozzi, Evan Meyer, and Jeffery Molkentin, PhD.
Postn+ Cardiac Fibroblasts in the Postnatal Heart
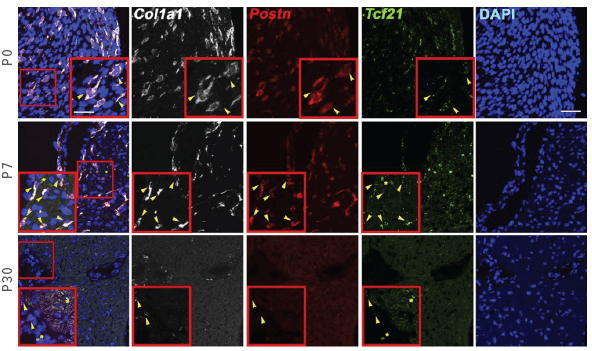
These rows depict RNAscope measurements of cells expressing Col1a1, Postn, and Tcf21 at 0, 7, and 30 days after birth in mice. Yellow arrowheads show positive colocalization of Postn, Tcf21, and Col1 confirming the fibroblast identity of these cells.




