Mirdametinib Shows Benefit Against NF1-Related Tumors in Phase II Trial
Published March 2021 | Journal of Clinical Oncology
Years of studying NF1 nerve tumors at Cincinnati Children’s continue to translate into medications that appear to show benefit for children and adults coping with painful, disfiguring and sometimes lethal plexiform neurofibromas (PNs).
Brian Weiss, MD, Nancy Ratner, PhD, and several colleagues here are part of the 15-center Neurofibromatosis Clinical Trials Consortium (NFCTC) funded by the U.S. Department of Defense Neurofibromatosis Research Program. Among their latest projects: a phase II clinical trial of the MAPK/ERK kinase inhibitor, mirdametinib.
Nineteen patients with PNs, ages 16-39, received up to 24 four-week courses of the medication. The consortium observed “significant and durable” pain reductions. They also reported that 42% of participants achieved partial tumor volume reductions by course 12, and 53% achieved stable disease. One patient developed progressive disease, despite treatment.
“To our knowledge, this analysis represents the first characterization of the activity and pharmacokinetics of mirdametinib in patients with NF1 and PNs and is the first published response study for MAPK/ERK kinase inhibitors in adults with NF1 and PNs,” according to Weiss, lead author of the study.
Cincinnati Children’s breakthroughs in NF1 research date back to 1992, when Ratner helped developed an important mouse model to study the genetic and molecular factors driving the condition. By 2012, Ratner and colleagues demonstrated that MEK inhibitors shrunk NF1 tumors in mice, and in human cells under lab conditions. By 2018, the drug selumetinib had received orphan drug approval from the U.S. FDA as the first treatment for NF1.
Weiss has played central roles in several clinical studies of NF1 treatments.
Mirdametinib Responses
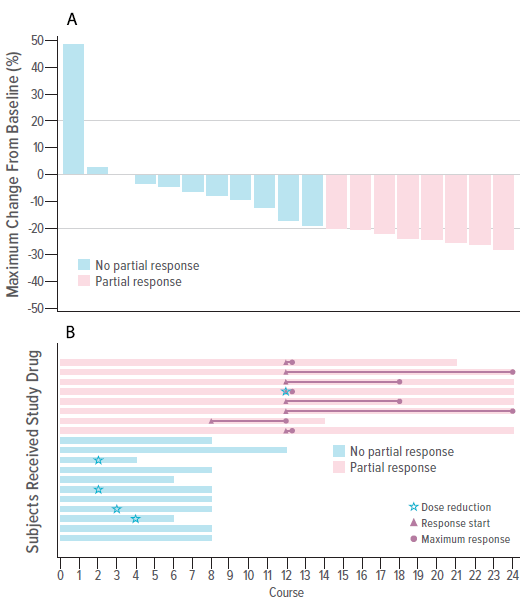
Each patient is represented by a single bar. Blue bars did not achieve a partial response (PR). Red bars achieved a PR. (A) Waterfall plot of maximal tumor volume change by patient. (B) Swimmers plot of duration of exposure, time to PR, and time to maximum response. Length of bar represents duration of exposure. Magenta triangles represent the time a PR was first observed, and magenta circles represent the time of maximum tumor volume change from baseline. Green stars represent time of dose reduction.





