Inotuzumab Leads to Remission in 58% of Children with Relapsed/Refractory B-ALL
Published March 2022 | Journal of Clinical Oncology
A recently published phase II clinical trial demonstrated that inotuzumab ozogamicin (InO) is safe and effective as a single-agent therapy in treating children and adolescents with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
The study’s principal investigator, Maureen O’Brien, MD, MS, director of the Leukemia/Lymphoma Program at Cincinnati Children’s, also is co-principal investigator for a second study that will evaluate InO’s safety and effectiveness when combined with chemotherapy in treating newly diagnosed high-risk B-ALL. Both studies are sponsored by Children’s Oncology Group.
InO is a CD22-targeting antibody-drug conjugate approved by the U.S. Food and Drug Administration to treat adults with relapsed or refractory B-ALL. CD22 is a surface protein expressed on leukemic blasts in about 95% of B-ALL cases.
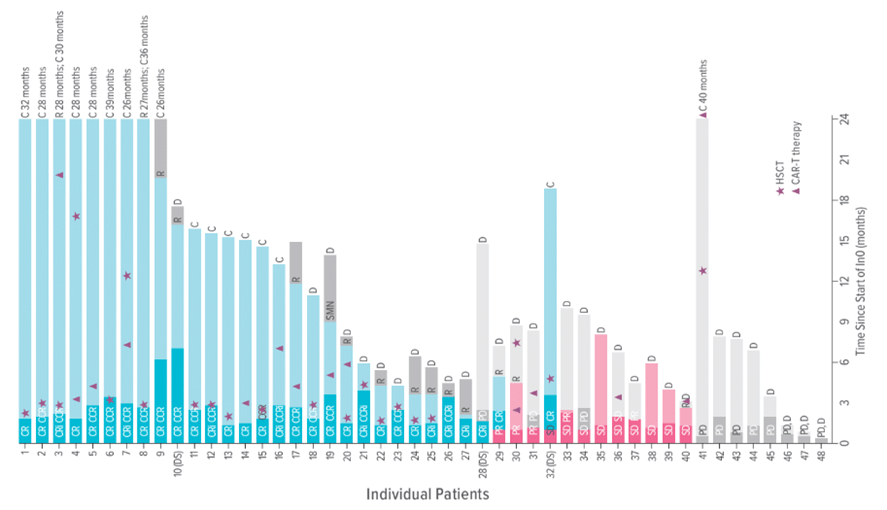
The phase II study of single-agent InO in relapse included 48 heavily pretreated patients ages 1 to 21 years with CD22-positive relapsed/refractory B-ALL.
“The patients who participated in this trial had few avail-able treatment options; many had received multiple intensive chemotherapy regimens and/or immunotherapies and had subsequently relapsed or did not respond,” says O’Brien. “InO as a single agent led to remission in 28 patients (58.3%), many of whom went on to receive subsequent curative therapy—either hematopoietic stem-cell transplantation (HSCT) or CAR-T (chimeric antigen receptor T cell) therapy.”
The phase II trial is enrolling a second cohort of patients with relapse who are receiving InO in combination with chemotherapy. The phase III study for newly diagnosed patients will enroll about 3,700 patients ages 1 to 25 years at 213 cancer centers in the US, Canada, Australia and New Zealand over the next four years.