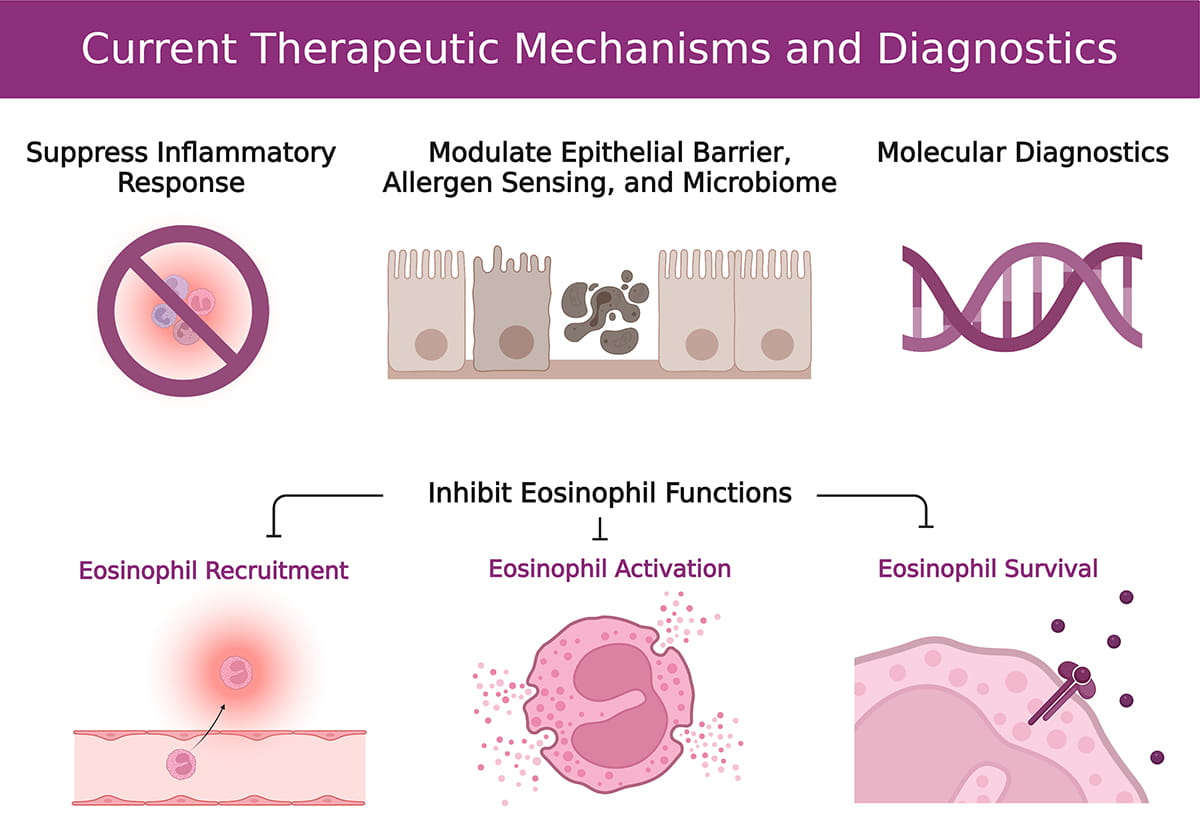
Eosinophilic Research
The Cincinnati Center for Eosinophilic Disorders (CCED) is a leader in research for these often-misunderstood conditions. Our research spans all stages of therapeutic development.
Stages of Therapeutic Development (*Level of CCED Involvement)
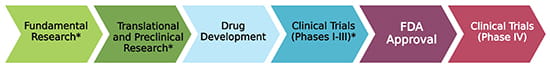
Developing new treatments and cures is an involved process that requires significant time and investment, especially during the fundamental stages of basic research and discovery validation, which are a major priority of the CCED. The CCED has a critical role in this process(*), working tirelessly on each stage, and has already had a key role in the development of therapeutic strategies for eosinophilic disorders such as eosinophilic esophagitis (EoE) and hypereosinophilic syndrome (HES).
Blocking Interleukin 13: An Example of the CCED Difference
Basic research leads to new discoveries essential for developing novel, viable therapeutics to treat human disease. For instance, a new therapeutic strategy for the treatment of EoE, Dupilumab, that blocks interleukin-13 (IL-13) signaling is now FDA for EoE for ages 1 year and older. The current anti-IL-13 antibody-based drugs being tested by biopharmaceutical companies, such as Novartis QAX576 and Celgene Corporation Cendakimab (formerly called RPC4046) and Sanofi and Regeneron Pharmaceuticals, Inc. Dupilumab, can be attributed to the research discoveries made at the CCED over the past decade.
Research Discoveries from the CCED that Provided the Rationale for Anti-IL-13

In 2003, Rothenberg et al. determined that administration of IL-13 could induce experimental EoE in mice, indicating its key role in this barely characterized disease. In 2005, Rothenberg et al. demonstrated that blocking IL-13 could reduce esophageal eosinophilia in mice, providing proof-of-principle of the utility of blocking IL-13 for treating inflammatory disease and esophageal eosinophilia. In 2006, Rothenberg et al. identified the EoE molecular signature and in 2007 showed that IL-13 could partially induce this EoE molecular signature, as well as establishing the increased levels and importance of IL-13 in human EoE. In 2010, Rothenberg et al. established that EoE in mice had similar pathologic characteristics and gene expression changes to EoE in humans and identified the involvement of the IL-13 receptor in the disease process.
Our subsequent partnership and discussion with pharmaceutical companies (e.g., Novartis, Receptos, Sanofi, and Regeneron) initiated the anti-IL-13 clinical trials. We contributed to the phase III trial of Dupilumab (Sanofi and Regeneron Pharmaceuticals, Inc.) for EoE in adolescents and adults. Trial data released in September 2020 suggested that Dupilumab could reduce symptoms and inflammation in patients 12 years and older with EoE, leading to Priority Review of Dupilumab for EoE by the FDA. We contributed to a phase III trial of Cendakimab (formerly RPC4046) for EoE that was initiated in 2021. The FDA approved Dupilumab for treatment of EoE for individuals 12 years and older in May 2022 and 1 year and older in January 2024.
All of these advancements in basic research and discovery were critical to the conception and development of blocking IL-13 as a therapeutic strategy for human EoE and would not have been possible without investment in fundamental research.
For research inquiries, contact the CCED at cced@cchmc.org.