Current Projects
Human papillomaviruses (HPVs) are the primary cause of cervical cancers, and have been associated with a subset of head and neck as well as other cancers. There is currently no effective antiviral therapy for an existing HPV infection, nor is there any way to predict which infections are likely to progress to cancer.
Since only a very small percentage of viral infections lead to cancer, it is critical to develop ways to diagnose, treat and predict the outcome of HPV infections. The goal of this research is to characterize the global program of gene expression during HPV-induced carcinogenesis. We have recently performed a meta-analysis of microarray expression data of clinical samples to identify gene expression changes associated with cervical tumors.
In particular, we are interested in how the molecular expression pattern changes during progression from an HPV-infected cell to a cancer cell. Using this data we are generating a comprehensive map of the molecular networks and pathways that underlie the process of HPV-induced carcinogenesis. In addition, we are using HPV-infected cells in vitro to identify the function and relative importance of the identified pathways, and design new therapies to inhibit HPV carcinogenesis.
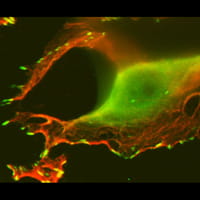
The human DEK protein is frequently upregulated in aggressive human tumors including squamous cell carcinoma, breast cancer and melanoma. A number of DEK functions have been described in vitro and include chromatin topology and nucleosome assembly, DNA replication, transcription, DNA repair and splicing. We have demonstrated that DEK suppresses senescence, apoptosis and differentiation in different model systems, thus promoting cellular growth and survival.
Furthermore, DEK exhibits oncogenic functions in 3-D models of cancer:
- First, DEK overexpression in human keratinocytes caused hyperplasia in organotypic epithelial rafts, with inappropriate cell cycle progression and expansion of the p63- positive stem and progenitor cell compartment.
- Second, DEK cooperated with known oncogenes for increased squamous cell tumor formation in immunodeficient mice.
- Third, DEK knockout mice exhibit resistance to skin, breast, and head and neck tumor development
Our studies are the first to define DEK as a bona fide oncogene, and current projects are focused on defining the underlying mechanisms of DEK function as an oncogene in epithelial cancers, and to interfere with DEK activities for the development of novel cancer therapies.
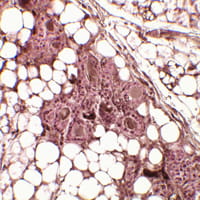
Fanconi anemia (FA) is a rare, autosomal recessive genome instability syndrome in which patients are at a dramatically elevated risk of squamous cell carcinoma (SCC) of the head and neck and anogenital tract.
Tumors appear early in life, and progress with striking aggressiveness. Conventional clastogenic therapies such as radiation and chemotherapy are often toxic because of the patients’ DNA damage response defects. Development of alternative treatments has been severely limited by the lack of availability of human models of FA SCC.
The FA Comprehensive Care Center at Cincinnati Children’s Hospital Medical Center has offered a unique opportunity for translational studies, and resulting patient-derived 3-D SCC models will be exploited to facilitate screens for new therapies and support preclinical trials for new treatments.
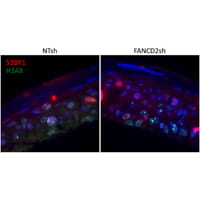
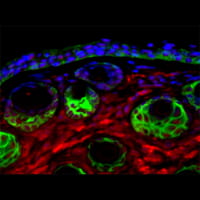
Our recent data demonstrate that loss of function of the FA pathway in high-risk HPV16 positive keratinocytes stimulates viral and cellular replication in vitro, and malignant transformation in vivo.
Based on these findings, we hypothesize that FA genes are modifiers of HPV infection. Experiments to determine the clinical and molecular importance of FA-HPV crosstalk are currently ongoing.
Contact Us
Susanne Wells, PhD
3333 Burnet Ave.
MLC 7015
Cincinnati, OH 45229-3039
Phone (S. Wells): 513-636-5986
Phone (Laboratory): 513-636-5967
Fax: 513-636-2880