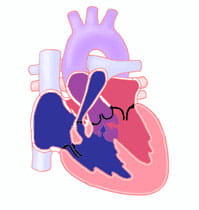
What is the Prognosis for Children With Tetralogy of Fallot?
Survival of children with tetralogy of Fallot has improved dramatically. In the absence of additional risk factors, more than 95% of infants with tetralogy of Fallot successfully undergo surgery in the first year of life.
Surgical repair is more difficult when the pulmonary arteries are critically small or when the lung blood flow is supplied predominantly by aortopulmonary collaterals.
Most babies are fairly sick in the first few days after surgery, since the right ventricle is "stiff" from the previous hypertrophy (thickness) and because an incision is made into the muscle of the ventricle, making the muscle temporarily weaker.
This right ventricular dysfunction usually improves significantly in the days following surgery. Patients may also have rhythm problems after surgery.
An abnormally fast rhythm (called junctional tachycardia) may occur and may require treatment with medication or the use of a temporary pacemaker. This abnormal rhythm is usually temporary and the rhythm generally will return to normal as the right ventricle recovers.
Patients are also at risk for slow heart rates after surgery due to heart block. Heart block may be caused by injury to or inflammation of the conduction system in the heart. In many patients, the conduction improves and normal rhythm returns. Rarely, a permanent pacemaker may be necessary.
Since normal circulation is produced by the tetralogy of Fallot repair procedure, long-term cardiac function is usually excellent.
However, the repair usually leaves the child with a leaky (insufficient) pulmonary valve. In this situation, after the right ventricle pumps blood out to the pulmonary arteries, some of the blood will flow back into the right ventricle. This creates extra volume in the right ventricle forcing it to work harder and become dilated.
In a small percentage of children, this pulmonary insufficiency can lead to diminished function of the right ventricle. Symptoms of fatigue, especially with exercise, may develop. In these cases, replacement of the pulmonary valve is often recommended, typically in the teenage years or in young adulthood.
Patients who have had repair of tetralogy of Fallot can also redevelop a narrowing in the right ventricular outflow area or in the branch (left or right) pulmonary arteries, which will cause the right ventricle to pump at abnormally high pressures.
If these problems occur, additional surgical intervention to further widen the outflow tract or pulmonary arteries may be necessary. Narrowing of the pulmonary arteries can sometimes be treated without surgery, with balloon dilation of the vessels during cardiac catheterization.
Long-term follow up with a cardiologist to detect recurrent or new problems as early as possible is essential. Follow-up visits in the cardiology clinic usually consist of a physical examination, electrocardiogram and periodic echocardiography. In addition, these visits will also include occasional cardiac MRI scans, exercise stress tests and Holter evaluations as a child reaches the teenage and adult years.