Epigenomics of T cell Memory
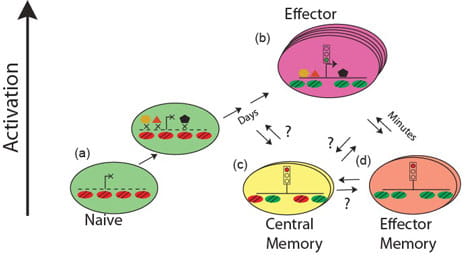
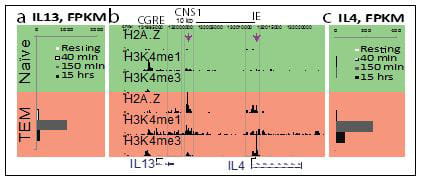
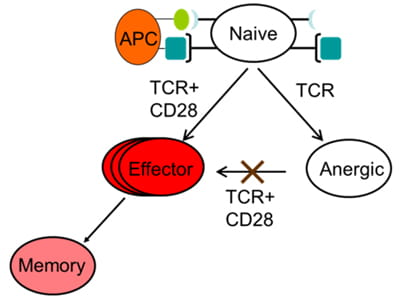
The goal of this project is to understand the role of chromatin and gene poising in T helper (Th) cell differentiation and memory T cell function. Upon initial antigen stimulation, naïve T cells may differentiate into several lineages of effector T cells. The heterogeneity within the CD4+ effector/memory T cell compartment is critical for our ability to deal with diverse pathogens. For example, dedicated populations of CD4+ Th cells are required for promoting immune defense against intracellular infections (Th1 cells), helminth infections (Th2 cells) and fungal infections (Th17 cells). However, each of these differentiated states is also associated with human disease: Th1 and Th17 cells can promote autoimmunity, whereas Th2 cells can promote allergy and asthma. Thus, understanding and learning to exploit the mechanisms that underlie lineage choice and lineage maintenance is vital for understanding and treatment of immunological and infectious diseases. We are interested in epigenetic regulation of T cell lineage commitment and T cell memory.