Current Projects
Current Projects
Regulatory T cells (Tregs), a subset of CD4+ T cells, are required both to establish and sustain immunological self-tolerance, as well as to quell exacerbated inflammation. My lab has studied Treg biology in several settings, notably chronic infection by HIV (Cutting Edge JI, 2005; Blood, 2006; J Virol, 2007, PLoSOne, 2011, and 2013, Blood, 2012; Front Immunol, 2014), aging (JI, 2008; JI, 2011; Front Immunol, 2013; JI, 2015; PLoS Pathogens, 2017) and pre-natal inflammation (see below). We also conducted collaborative studies of Treg function and differentiation (Science, 2015; Sci Reports, 2017). To better study human Treg function, we have developed advanced multiparametric phenotypic strategies (Fig. 1) and assays that probe Treg suppressive action on dendritic cells and their capacity to activate conventional T cells, using imaging flow cytometry (Front Immunol, 2014).
Recently, our lab has focused on studying the interactions between Treg and high-density lipoproteins (HDL). We found that HDL levels are associated with Treg numbers in persons taking statins (J Immunol Res, 2015). This data prompted our studies of the underlying mechanisms for such association, and we uncovered the previously unrecognized direct interactions between HDL and Tregs. We indeed showed that HDL selectively bind to Tregs, which increases Treg mitochondrial oxidative metabolism (J Lipid Res, 2017) and decreases their caspase-dependent apoptosis (J Lipid Res, 2023). We recently found that HDL interact most with the memory Treg subsets, particularly with the highly suppressive effector memory Treg subset. We also identified the key role of apolipoproteins, specifically apolipoprotein E, in this effect (J Lipid Res, 2023). This newly identified mechanism likely contributes to the atheroprotective role of HDL. Importantly, we have found decreased frequency of memory Treg in HIV-infected patients with high risk of cardiovascular disease (CVD), which correlated with their defective anti-inflammatory response to HDL (Front Immunol, 2023). We are now focusing our studies on the underlying mechanisms, notably analyzing the pathways involved in Treg-HDL interactions and their anti-apoptotic effect, as well as analyzing these dynamic interactions in a longitudinal cohort of obese adolescents before and after bariatric surgery. We also continue to explore Treg response to HDL in HIV-infected patients, in relation to their CVD risk.
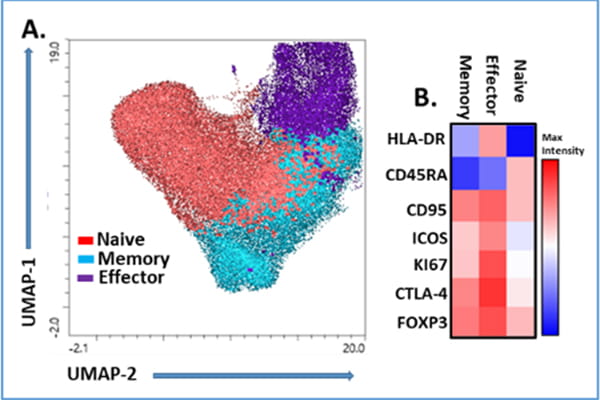
Fig. 1: Analysis of human Regulatory T cell phenotype (from Front Immunol, 2023).
We developed high multiparametric flow cytometry panels to phenotype Treg heterogeneity and functionality. Circulating Treg subsets were analyzed in PBMC from healthy controls by flow cytometry. The unbiased clustering by (A) UMAP (B) FlowSOM identified three main subsets after gating in the CD3+CD4+CD25+FOXP3+ cells. According with the expression of CD45RA, CD95, HLA-DR, ICOS, CTLA-4, Ki67 and FOXP3, these were classified as naïve, memory and effector Treg.
Collaborators:
This HDL-Treg project is conducted in close collaboration with Dr W.Sean Davidson at the University of Cincinnati and Dr. Amy Shah in the Division of Endocrinology. Studies of HIV infected individuals are done in close collaboration with Dr. Carl J. Fichtenbaum and Dr. Moises A. Huaman at the University of Cincinnati.
Aftermath of prenatal inflammation and its alleviating strategies
For over a decade, my lab has been studying how prenatal exposure to inflammation, especially chorioamnionitis (HCA), shapes the developing fetal immune system (Fig. 2). Despite its significance (this concept is key in the “Developmental Origins of Health and Disease (DOHaD)” theory)”, many gaps in knowledge remain in this field. We have studied samples from human cohorts as well as developed relevant non-human primate (NHP) models to address these important questions.
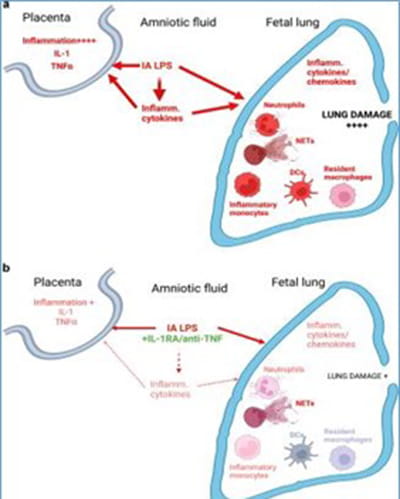
Fig. 2 Schematic representation of mechanisms involved in fetal lung damage (from Mucosal Immunol, 2022).
Our main findings encompass several facets:
1. Systemic inflammation
The key cell type regulating immune responses are regulatory T cells (Tregs), a subset of CD4+ T cells expressing the transcription factor FOXP3. We have shown that prenatal inflammation, particularly severe HCA, impacts both Treg numbers and their phenotype (J Immunol, 2013; Hum Immunol, 2015; J Immunol, 2016; J Infect Dis, 2016; Pediatr Res, 2017). Also supporting the concept that fetal exposure to inflammation has systemic effects, we showed that enhanced levels of pro-inflammatory mediators, including IL-1β, CCL2 and COX-2 can lead to pathological changes in fetal microglia and white matter (J Neuroinflam, 2016).
2. Mucosal inflammation
Lungs are the major organ affected by placenta inflammation, as they are in direct contact with the amniotic fluid. In our NHP model (intra-amniotic LPS), we showed massive inflammatory cell recruitment, particularly of myeloid cells (Mucosal Immunol, 2022) and disruption of structural integrity and key signaling networks in the developing alveolus (Sci Transl Med, 2022). These collaborative mechanistic studies in relevant animal models provide a better framework to understand why exposure to severe HCA, but not mild HCA, is independently associated with wheeze and respiratory-related physician visits in the first year of life (Ann Am Thorac Soc. 2016).
3. Placenta inflammation
In collaborative studies done in different NHP models of inflammation and in human cohorts, we also analyzed immune cell recruitment and signaling pathways in chorioamnion-decidua tissues (Biol Reprod, 2015; JCI Insight, 2018; Front Immunol, 2020). Notably, our data comparing intra-amniotic LPS vs. E. coli, showed that the intensity of the host immune response to intrauterine infection/inflammation may determine susceptibility to preterm labor (PLOS Biology, 2021).
4. Role of cytokines in HCA-induced fetal inflammation.
HCA, particularly severe HCA, is characterized by the presence of high levels of pro-inflammatory cytokines in the amniotic fluid, and on the fetal side, locally (in the lung alveolar wash) and systemically (in the plasma). To determine the role this altered cytokine milieu plays in driving lung damage and systemic inflammation, we tested the effect of anti-inflammatory blockades (IL-1R antagonist and anti-TNFα antibody, alone or combined) in our NHP model. While these blockades did not prevent the inflammatory cell recruitment in the lung, they blunted the lung overall inflammatory state (Mucosal Immunol, 2022) leading to restoration of structural integrity and key signaling networks in the developing alveolus (Sci Transl Med, 2022). In addition, blocking either IL-1 or TNFα pathway decreased accumulation and activation of neutrophils in the placenta (JCI Insight, 2018; Front Immunol, 2020). Blockade of the IL-1 pathway also reverted some of the systemic alterations, such as Treg dysfunction (JI, 2016).
5. Effect of antenatal corticosteroids (ACS)
Administration of antenatal corticosteroids (ACS) to attenuate some of the respiratory complications of pre-term birth is a routine procedure in at risk pregnancies. However, our collaborative studies in NHP model indicate ACS impact neuronal development (JCI insight, 2022) and, in the context of inflammation, trigger transcriptomic response that increases the risk of abnormal extracellular matrix development in the lung (JCI Insight, 2020). Ongoing collaborative studies in our NHP model, combining single cell genomics, multi-parameter flow cytometry and immuno-histochemistry) are examining the impact of ACS alone, or in the context of intrauterine infection/inflammation, on fetal organs and immune responses.
Collaborators:
This project has been conducted in close collaboration with Dr. William Zacharias, MD, PhD, Dr. Alan Jobe, MD, PhD, Dr. Louis Muglia, MD, PhD and Dr. Hitesh Deshmukh, MD, PhD in the Perinatal Institute, Dr. Ian Lewkowich, PhD in the Division of Immunobiology, Dr. Suhas Kallapur, MD at UCLA, and Dr. Lisa Miller, PhD and Dr. John Capitanio, PhD at the California National Primate Research Center in Davis, CA.
Over 50% of very preterm births have an antecedent infection associated with chorioamnionitis or premature rupture of membranes. Pathological investigations suggest an initial focal choriodecidual colonization by lower genital tract organisms (including Ureaplasma species, commonly associated with preterm birth) followed by dissemination into the amniotic fluid, and induction of preterm labor. Concurrently, the fetus is exposed to bacteria, bacterial products (such as LPS) and/or inflammatory cytokines, causing fetal inflammatory response syndrome (FIRS). However, the precise mechanisms of how microorganisms trigger inflammatory changes (in the placenta and fetus) remain poorly defined.
We are actively pursuing this line of research, principally using nonhuman primate models of experimental chorioamnionitis to characterize fetal (JI, 2013) or placental inflammation (Biol Reproduction, 2014).
Collaborators: This project is conducted in collaboration with Alan Jobe, MD, PhD, Suhas Kallapur, MD, and Louis Muglia, MD, PhD, in the Perinatal Institute, and Lisa Miller, PhD and John Capitanio PhD at the California National Primate Research Center in Davis, CA.
Funding:
- NIH U01HL101800 (co-PIs: A. Jobe/C. Chougnet) Biomarkers of Immunologic Function and Preterm Respiratory Outcomes.
- NIH 1R01HD078127 (co-PIs: L. Muglia/C. Chougnet) Maternal temperament, stress, and inflammation in preterm birth.
- Burrough-Wellcome (PI: C. Chougnet) Host-microbe cross talk and pregnancy outcomes.
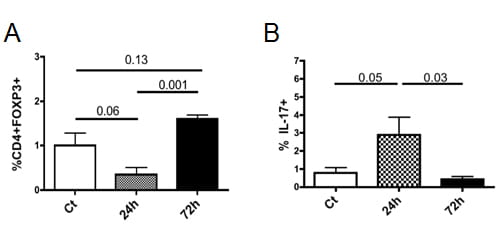
Intra-amniotic IL-1β transiently decreases regulatory T cell (Treg) frequency but increases the frequency of IL-17+ cells in lymphoid organs.
Human recombinant IL-1β, or saline, was injected intra-amniotically to pregnant rhesus macaques at ~80% gestation. At 24h or 48h post-injection, fetuses (n=4-6/group) were delivered by C-section and immune responses were analyzed in tissues (spleen and lymph nodes) by flow cytometry. Data from the spleen are shown. (A) Mean (SE) Treg frequency (defined as percentage of FOXP3+ cells in the gated CD3+CD4+ population). (B) Mean percentages of IL-17A+ cells in gated CD3+CD4+ cells were measured after short in vitro PMA and ionomycin stimulation. Significant p values (Mann-Whitney tests) or trends are shown. From Kallapur et al, JI, 2013.
Aging has a profound negative impact on the capacity of the immune system to develop efficient effector responses against a vast array of antigens, leading to poor response to pathogens or vaccines. Importantly, aging affects all arms of the immune system, but most of the research has so far focused on the adaptive immune response.
We have shown that Treg homeostasis is changed during aging, leading to increased survival and accumulation in lymphoid organs (JI, 2008; JI, 2011; Front Immunol, 2013). We are now deciphering the underlying mechanisms as Treg accumulation is an important contributing factor to immunosenescence.
Impaired functionality of dendritic cells (DC) is likely a significant element of this decreased response, and deciphering the underlying mechanisms has thus become an important area of investigation. In collaboration with Dr. Edith Janssen (Division of Immunobiology), we focus on this aspect of DC biology, linking age-related changes in mitochondrial functions with the impaired capacity of the aged DC to prime CD8+T cells to cell-associated antigens
Natural killer cells (NK cells) also have impaired maturation and function in aging. Our work, in collaboration with Dr. Kasper Hoebe suggests that the aged non-hematopoietic environment is responsible for these defects (Aging Cell, 2014). We are further defining these non-hematopoietic factors, as identifying them could open new therapeutic avenues to improve NK cell function in the elderly.
Collaborators: David Hildeman PhD, Kasper Hoebe PhD
Funding:
NIH R01 AG033057 (co-PIs: C. Chougnet/D. Hildeman) Homeostasis and function of regulatory T cells in aging.
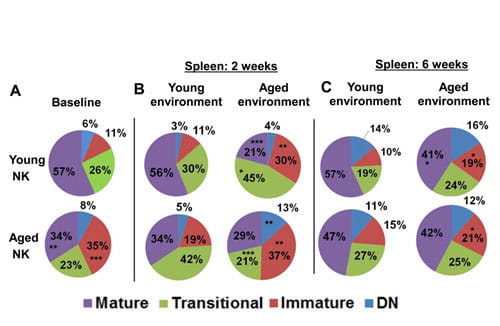
NK cell maturation is impaired and is controlled by non-hematopoietic factors
(A): Proportion of splenic NK subsets (double negative, immature, transitional and mature) in aged and young mice. p values represent the differences between young and aged mice. *P<0.05, **P<0.01 and ***P<0.005. (B) to (C): Proportion of splenic NK subsets at 2 weeks (B) and 6 weeks (C) post bone marrow chimera in a young or aged environment. p values represent the difference between NK cells maturing in a young vs. aged environment. From Shehata et al, Aging Cell, in press.



