Wells Lab Research
Research in the Wells lab aims to uncover the molecular and cellular mechanisms by which gastrointestinal and endocrine organs form in the developing embryo. While an understanding of embryonic organogenesis is far from complete, the Wells lab uses our current base of knowledge in attempts to improve child health in the following ways:
1) Embryonic development of endoderm organs: We identify the pathways that regulate organ development to uncover the genetic and environmental factors that cause deranged organ development with a focus on causes of diabetes and digestive diseases.
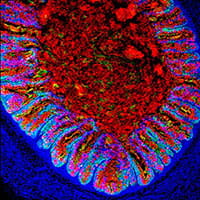 2) Generating human tissues from pluripotent stem cells: Through manipulation of pathways that control embryonic organ formation, we can direct the differentiation of pluripotent stem cells into human organ tissues called organoids that can be used to study human organ development and disease. Examples include gastrointestinal (GI) tissues (esophagus, stomach, intestine, colon) that are used to study malabsorption syndromes and infectious diseases of the GI tract and endocrine cells, including pancreatic beta cells that we use to study genetic forms of diabetes. We also use principles learned from embryonic organogenesis to engineer more complex and functional tissues from human pluripotent stem cells. For example, we have engineered the enteric nervous system into small intestinal organoids that drive the mechanical movements of peristalsis as well as colonic organoids with functional immune cells that mediate an inflammatory response to bacteria.
2) Generating human tissues from pluripotent stem cells: Through manipulation of pathways that control embryonic organ formation, we can direct the differentiation of pluripotent stem cells into human organ tissues called organoids that can be used to study human organ development and disease. Examples include gastrointestinal (GI) tissues (esophagus, stomach, intestine, colon) that are used to study malabsorption syndromes and infectious diseases of the GI tract and endocrine cells, including pancreatic beta cells that we use to study genetic forms of diabetes. We also use principles learned from embryonic organogenesis to engineer more complex and functional tissues from human pluripotent stem cells. For example, we have engineered the enteric nervous system into small intestinal organoids that drive the mechanical movements of peristalsis as well as colonic organoids with functional immune cells that mediate an inflammatory response to bacteria.
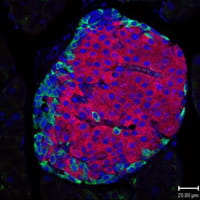 3) Organ function and dysfunction: Diabetes and digestive diseases. Using a combination of animal models and human tissue organoid models, we study how cells and tissues control endocrine and gastrointestinal functions. Moreover, we have developed several unique platforms to study the effects of genetic and environmental factors such as pathogens on human organ function as well as models to test the effect of drugs on organ function and to test the ability of engineered human tissues to restore organ function in animal models of disease such as Diabetes.
3) Organ function and dysfunction: Diabetes and digestive diseases. Using a combination of animal models and human tissue organoid models, we study how cells and tissues control endocrine and gastrointestinal functions. Moreover, we have developed several unique platforms to study the effects of genetic and environmental factors such as pathogens on human organ function as well as models to test the effect of drugs on organ function and to test the ability of engineered human tissues to restore organ function in animal models of disease such as Diabetes.
The long-term goal of this research is to generate healthy, therapeutic replacement tissues for children with diabetes and digestive diseases, and the Wells lab has active collaborations with surgeons, gastroenterologists and endocrinologists in order to drive this translational goal.








