Scientists Add Nerves to Intestinal Organoids
Research By: Michael Helmrath, MD, MS | James Wells, PhD | Samantha Brugmann, PhD
Post Date: June 30, 2019 | Publish Date: Jan. 23, 2017
“The ability to study human intestinal function fills a key gap in our understanding of many issues that result in significant morbidity in both children and adults.”
—Michael Helmrath, MD
Cincinnati Children’s is growing hope for children with complex gastrointestinal disorders. All it took was an ocean of research ambition and a dash of nerve.
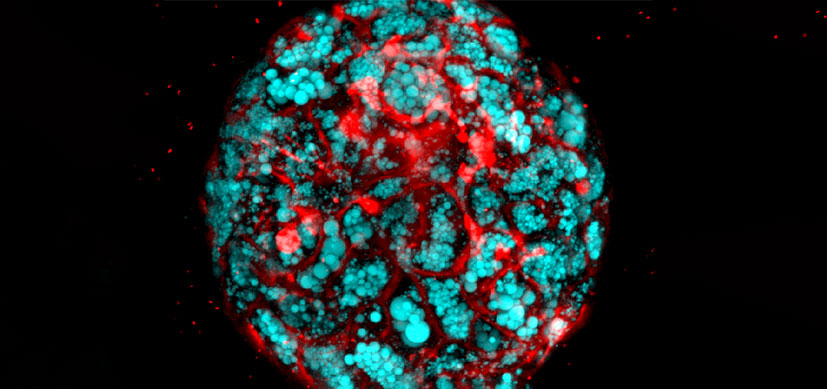
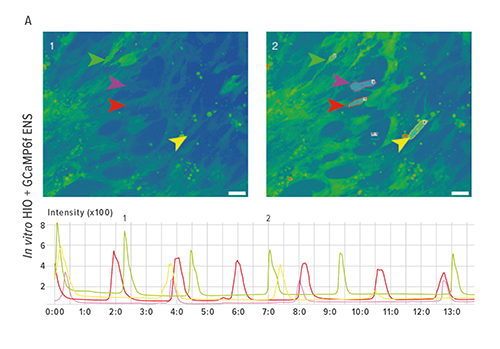
The study opened a new chapter in the book on regenerative medicine when researchers demonstrated how they grew human intestinal organoids with functioning enteric nerves in a petri dish, using human pluripotent stem cells (hPSCs).
These particular stem cells are stars because they can develop into any type of cell in the body.
This was, the team wrote, “to the best of our knowledge, the first demonstration of human-PSC-derived intestinal tissue with a functional enteric nervous system and how this system can be used to study motility disorders of the human gastrointestinal tract.”
It might be years before this development directly guides the treatment of individual children, or even leads to growing patient-specific human intestine for transplant.
But right now the team is using these organoids to recreate and study Hirschsprung’s disease, a severe congenital intestinal nerve disorder caused by a mutation in the gene PHOX2B, which results in missing nerve cells in the muscles of the colon.
ORGANOID ADVANCE FUELS INSIGHTS FOR HIRSCHSPRUNG DISEASE AND MORE
Reflecting on the impact following the study’s release, co-senior author Michael Helmrath, MD, emphasized the value of validation.
“The ability to study human intestinal function fills a key gap in our understanding of many issues that result in significant morbidity in both children and adults,” says Helmrath, Surgical Director at the Intestinal Rehabilitation Program. “The opportunity to see this first-hand is exciting, as it validates the potential we have to translate world-class basic science to the care of patients. That is our mission at Cincinnati Children’s.”
The team included faculty from the divisions of Pediatric General and Thoracic Surgery, Developmental Biology, Plastic Surgery, and Endocrinology. External partners included colleagues in San Francisco, France and Australia.
The potential for this new technology starts with being able to model and study intestinal disorders in functioning, three-dimensional human organ tissue with genetically-specific patient cells. This can allow researchers to test new therapeutics in functioning lab-engineered human intestine before clinical trials in patients.
“Many oral medications give you diarrhea, cramps and impair intestinal motility,” says James Wells, PhD, co-lead investigator with Helmrath and Director of the Pluripotent Stem Cell Facility at Cincinnati Children’s. “A fairly immediate goal for this technology that would help the largest number of people is as a first-pass screen for new drugs.”
ORGANOIDS MAY HELP AVOID SIDE EFFECTS IN DRUG DEVELOPMENT
The organoids provide a scientific flashlight that allows researchers to look for off-target toxicities and prevent side effects in the intestine, the body’s largest immune organ and primary interface with the outside world.
As science continues to learn more about how important intestinal health is to overall health, Wells and Helmrath say using functioning lab-generated human intestine creates an array of new research opportunities.
Some include the ability to conduct deeper studies into nutritional health, diabetes, severe intestinal diseases (like necrotizing enterocolitis), and biochemical changes in the body from gastric bypass weight-loss surgery, the latter being one of Helmrath’s specialties.
Since the study’s release, new data further support the key role the enteric nervous system plays on intestinal development and function.
“This is the foundation of studies,” Helmrath says, “to understand complex human conditions with aberrant motility that remain difficult clinical conditions to manage.”
A TALE OF TWO DISHES
To engineer a nervous system for the intestinal organoids already growing in one petri dish, researchers used a second dish to generate embryonic-stage neural crest cells, which were manipulated to form precursor cells for enteric nerves. The challenge was to identify how and when to incorporate the neural crest cells into the developing intestine.
“We tried a few different approaches largely based on the hypothesis that, if you put the right cells together at the right time in the petri dish, they’ll know what do to,” Wells says.
That’s where study co-author Samantha Brugmann, PhD, came in. A developmental biologist in the Division of Plastic Surgery, she provided critical insights into embryonic nerve development.
The key, the team knew, was to get the neural crest cells to cooperate in a scientifically meaningful way. Timing was everything.
“It was a longshot,” Wells says. “But it worked.”
| Original title: | Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system |
| Published in: | Nature Medicine |
| Publish date: | Jan. 23, 2017 |
Research By