Study Explores the Biology of Mending a Broken Heart
Study Explores the Biology of Mending a Broken Heart
Scientists Test Way to Stop Heart Damage After Cardiac Injury
Thursday, September 28, 2017
Early research results suggest scientists might be on to a way to preserve heart function after heart attacks or for people with inherited heart defects called congenital cardiomyopathies.
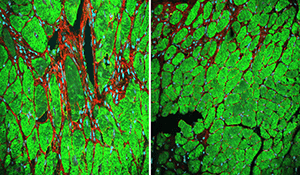
Researchers at the Cincinnati Children’s Heart Institute report Sept. 28 in Nature Communications that after simulating heart injury in laboratory mouse models, they stopped or slowed cardiac fibrosis, organ enlargement and preserved heart function by blocking a well-known molecular pathway.
The Wnt/β-catenin signaling pathway is involved in several of the body’s fundamental biological processes. After heart injury, however, Wnt/β-catenin signaling ramps up in cardiac fibroblast cells to cause fibrosis, scarring and harmful enlargement of the heart muscle, according to the researchers.
“Our findings provide new insights on what causes cardiac fibrosis and they open the potential for finding new therapeutic approaches to fight it and preserve heart function,” says Katherine Yutzey, PhD, lead study author and scientist in Molecular Cardiovascular Biology. “Wnt/β-catenin signaling is involved in many normal and disease processes and it’s tough to target therapeutically. But the idea that early targeting of fibrotic response in cardiac disease may improve muscle function and stop disease is an exciting new direction.”
Heart disease is one of the leading killers of people in the United States, according to the U.S. Centers for Disease Control. And a growing number of adults who were treated for congenital heart malformations as children – many who essentially had their hearts reconstructed – face lifelong potential health complications as a result.
Adult congenital heart disease is a growing priority and area of scientific inquiry at Cincinnati Children’s. Clinicians and researchers at the medical center are developing new clinical and research strategies for adults who were pediatric heart patients. This includes looking for what so far are elusive therapeutic strategies to repair scarred and poorly functioning heart tissues after cardiac injury or disease.
Mice Help Show the Way
In the current study, Yutzey and her colleagues used a newly developed line of genetically bred laboratory mice that allowed them to determine how important Wnt/β-catenin signaling is in cardiac fibroblast cells. Fibroblasts are important to building the connective tissues and structural framework cells that help hold the body together. But in the context of heart disease, researchers are learning resident cardiac fibroblast cells cause a deadly mix of tissue fibrosis, scarring and diminished function.
To simulate cardiac injury in the mice, researchers conducted a procedure called trans-aortic constriction to restrict blood flow through the heart. Some of the mice were bred so that following cardiac injury they did not express cardiac Wnt/β-catenin in fibroblasts. Control mice in the study continued to express Wnt/β-catenin following heart injury.
The control mice exhibited extensive fibrosis, scarring and diminished heart function. Mice not expressing Wnt/β-catenin had diminished fibrosis and scarring and the animals’ heart function was preserved.
Study authors stress the laboratory research is still at an early stage and it is too early to know to what extent it might apply to actual clinical treatment. But the findings of this study, and other papers involving different molecular pathways by other research teams, have intensified interest in understanding how inhibiting cardiac fibrosis improves muscle cell function and pathology.
This will be a central question in future research heart fibrosis studies by Yutzey and her colleagues, along with looking for possible ways to target the Wnt/β-catenin signaling pathway for potential therapeutic benefit.
Funding support for the study came from the National Heart Lung and Blood Institute of the National Institutes of Health (P01HL069779) and an American Heart Association fellowship to study co-authors Fu-Li Xiang, PhD, and Ming Fang, both members of Yutzey’s laboratory team.