Fragile X syndrome: New drug improves behavior symptoms in mouse model
Fragile X syndrome: New drug improves behavior symptoms in mouse model
Friday, July 13, 2018
New research in mice shows that a drug similar to one under evaluation as a possible cancer treatment eventually might also help people born with fragile X syndrome (FXS), the most common inherited form of intellectual disability and a major cause of autism spectrum disorders.
FXS affects about 1 in 3,000 boys and 1 in 6,000 girls, with boys generally experiencing more extreme symptoms. Affected children often display delayed speech development, impaired social skills, gaze aversion, social anxiety, hyperactivity and repetitive behaviors.
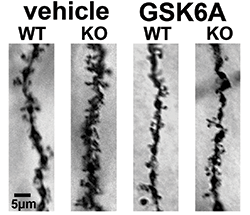
The syndrome is caused by the silencing of a single gene, FMR1. This results in an overabundance of synaptic structures within the brain that disrupt normal communication between neurons.
Early-stage testing shows promise
However, a team of scientists from Cincinnati Children’s and the Emory University School of Medicine report that an enzyme-inhibiting compound made by GlaxoSmithKline can prevent the excessive synaptic growth and relieve several symptoms of FXS.
The team’s findings are scheduled for publication in Neuropsychopharmacology, and are being presented today at the NFXF International Fragile X Conference in Cincinnati.
When the compound GSK6A was given to mice lacking the Fmr1 gene, an established animal model of the syndrome, it relieved symptomatic behaviors, such as impaired social interactions and inflexible decision making. These symptoms often occur among people with FXS.
The compound inhibits a signaling enzyme called p110β, one of four forms of PI3 (phosphoinositide-3) kinase. A closely related p110β inhibitor is already in clinical trials for cancer.
“Our results suggest that p110β inhibitors can be repurposed for fragile X syndrome, and they have implications for other subtypes of autism spectrum disorders that are characterized by similar alterations of this pathway,” says Gary Bassell, PhD, professor and chair of cell biology at Emory University School of Medicine.
The paper represents a collaboration between three laboratories: two at Emory led by Bassell and Shannon Gourley, PhD (Gourley is based at Yerkes National Primate Research Center), and one at Cincinnati Children’s, led by neuroscientist Christina Gross, PhD.
“Right now, no proven efficient treatments are available for fragile X syndrome that are targeted to the disease mechanism,” Gross says. “We think that p110β is an appropriate target because it is directly regulated by FMRP, and it is overactivated in both mouse models and patient cell lines.”
One part of the study explored how GSK6A affected social interaction. Given a choice between spending time in a chamber with an unfamiliar mouse or in another chamber with an object, normal mice will prefer a living companion, but mice mimicking FXS show no preference. Treating these mice with GSK6A one hour before the test increased their social preference, the study reports.
The new study also went beyond mouse behavioral tests to include molecular, cellular and physiological data that support the findings.
“It is likely that PI3 kinase – in its various forms -- needs to be kept in a tight balance at the synapse,” Bassell says. “Too much or too little can tip things into a suboptimal zone, especially for complex behaviors. We think targeting the excess p110β in FXS can restore signaling between upstream and downstream defects linked to the absence of FMRP.”
More work needed before clinical trials can start
The FXS community has seen hopes raised and dashed by other compounds that showed promise at the mouse-testing stage. The researchers involved in this study emphasize that their findings remain too preliminary to begin human testing.
For example, the mouse studies described in the paper cover short-term treatment (one or two injections). Longer-term animal studies are needed to measure effectiveness and to detect potential side effects before human clinical trials to evaluate FXS effects can begin.
About the study
This research was supported by the National Institute of Mental Health (R21MH103748), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54HD082013), Office of Research Infrastructure Programs (Primate centers: P51OD011132), the Brain & Behavior Research Foundation, the Cincinnati Children’s Research Foundation, and Children’s Healthcare of Atlanta.
Gross and Bassell have applied for a provisional patent based on this technology, and thus could potentially benefit from its commercialization.
Contact Information
Jim Feuer
513-636-4656