Cincinnati Children’s seeks volunteers for AstraZeneca COVID-19 vaccine trial
Cincinnati Children’s seeks volunteers for AstraZeneca COVID-19 vaccine trial
Center reaching out to Blacks, Hispanics, seniors and first responders
Tuesday, November 03, 2020
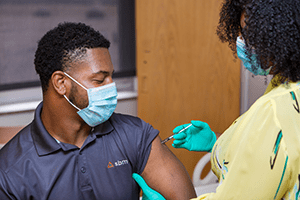
Cincinnati Children’s intends to enroll about 500 people in a Phase 3 clinical trial of a COVID-19 vaccine being developed by AstraZeneca.
The medical center is reaching out to first responders, people 65 or older, Blacks and Hispanics to ensure inclusion of those at higher risk of contracting the disease or becoming seriously ill.
David Bernstein, MD, is principal investigator for the part of the study being conducted at Cincinnati Children’s. AstraZeneca is sponsoring the trial at more than 60 sites in the U.S.
Bernstein, professor of pediatrics in the Division of Infectious Diseases at Cincinnati Children’s, said the local part of the study will begin in November by enrolling volunteers 18 or older. Two shots will be administered 28 days apart to each participant. They will be injected with either the AstraZeneca vaccine AZD1222 or a saline placebo.
“One of our goals is to have a large representation of minorities – Hispanics and Blacks – because they’ve been harder hit,” Bernstein said. “We’re looking for people at higher risk of exposure or more likely to get ill. We are going to heavily enroll the elderly, minorities and first responders – including healthcare workers.
“I reached out to fire departments and police,” Bernstein added. “They are interested. We will use our Mobile Clinic to go out to a local police or fire station for vaccination if we can enroll a good number.”
No infection risk for volunteers
There is no risk that the vaccine will infect a participant, said Bernstein, who noted that AZD1222 contains only one of the many COVID proteins – but not the virus that causes illness. The protein prompts an immune response.
“There is absolutely no chance of getting COVID (from the vaccine),” Bernstein said. “We need to do something to end this pandemic. I think these vaccines will play a big role in doing that.
“It’s a larger trial than almost anything we’ve ever done, but these are different times,” said Bernstein, who is also associate director of the Gamble Vaccine Research Center at Cincinnati Children’s, which is one of the world’s leading institutions for vaccine research. “It’s not easy if you need to enroll hundreds. We’ve been doing this for over 30 years, so we’re relying on our experience.”
Up to 30,000 people will participate in the U.S. trial across the various sites. The AstraZeneca trial is part of Operation Warp Speed, a federal effort to accelerate the development, manufacture, and distribution of vaccines to prevent the spread of COVID-19.
Cincinnati Children’s is part of the COVID-19 Prevention Network (CoVPN). The National Institutes of Health-supported network, which is composed of existing clinical research networks with infectious disease expertise, is designed for rapid and thorough evaluation of vaccine candidates and monoclonal antibodies for the prevention of COVID-19.
How to participate
Adults interested in joining the local study may call Cincinnati Children’s at 513-636-7699, email gambleprogram@cchmc.org or follow this link.
Participants in the local trial will be compensated for their time, which would include visits as well as recording their temperature, any local pain, head aches or other mild side effects after each shot. Participants will provide blood and have COVID testing done at the initial visit. They will also provide blood samples periodically.
Funding for the trial is provided by the National Institute of Allergy and Infectious Diseases (part of the National Institutes of Health) and the Biomedical Advanced Research and Development Authority (part of the U.S. Department of Health and Human Services).
The trial is designed primarily to determine if AZD1222 can prevent symptomatic COVID-19 after two doses, according to the NIH.
Oxford University’s Jenner Institute and Oxford Vaccine Group developed AZD1222. The candidate vaccine was licensed to AstraZeneca for further development.
For more information about the study, visit ClinicalTrials.gov.
Contact Information
Barrett J. Brunsman
barrett.brunsman@cchmc.org