Lab Projects
The Erickson Lab is NIH-funded and actively recruiting members to join us on several exciting projects that intersect the fields of immunology, glycobiology and reproductive biology.
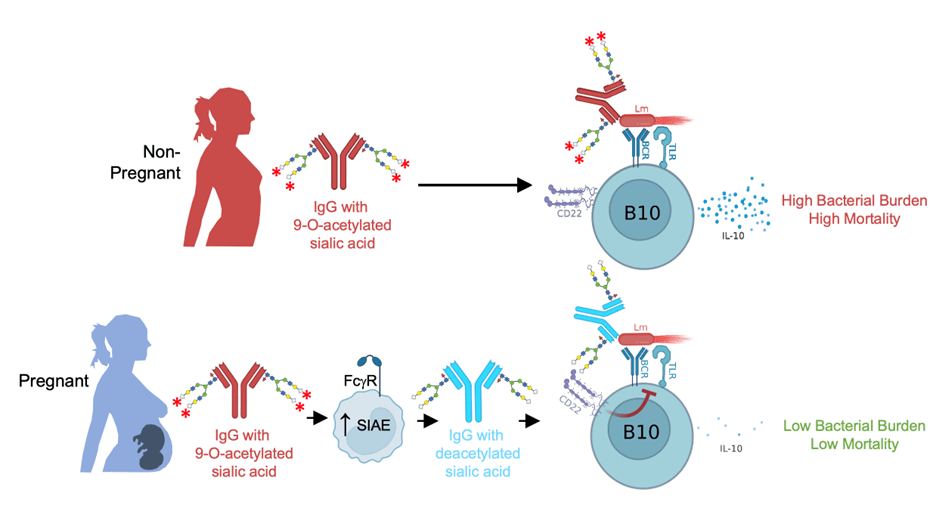
Sialylated Antibody Defense Against Intracellular Pathogens
Division of labor between humoral and cellular adaptive immune components for effective host defense is a foundational immunological tenet. Antibodies are thought to offer limited protection against intracellular pathogens since they cannot efficiently cross the plasma membrane to enter most cells, which may explain the prevalence of intracellular infections in fetuses and newborns given their dependence on vertically transferred maternal IgG for early life immunity. However, we recently demonstrated that pregnancy enables antibody-mediated protection against the prototypical intracellular pathogen Listeria monocytogenes (Lm). The key molecular change during pregnancy is deacetylation of sialic acid (Sia) located in the terminal position of N-glycans on preconceptually primed Lm-specific IgG. Maternal deacetylated IgG then modulates neonatal B cells via the Sia receptor CD22, which can only bind to deacetylated Sia. This establishes a framework whereby differential acetylation versus deacetylation of sialylated IgG serves as a previously unrecognized molecular “switch” that greatly impacts immunity through CD22, which in turn controls responder B cell activation and immunomodulatory functions. We are actively investigating the factors that lead to the generation of antibodies bearing acetylated sialic acid, the cells and enzymes that remodel sialic acid during pregnancy, and the coordinated activity of antibodies, cells and cytokines that mediate host defense against neonatal infection.
Maternal B Cells and Fetal Tolerance
Pregnancy involves a complex interplay between the immune system and tissues of the mother and developing fetus. Diverse immunological adaptations are required to prevent rejection of genetically foreign fetal tissues. Prior research has focused on maternal immune suppressive CD4+ Foxp3+ regulatory T cells (Treg) as drivers of fetal tolerance. More recently it was shown that maternal B cells can suppress proliferation of effector T cells with fetal specificity. Whether maternal B cells enforce fetal tolerance, and their precise mechanisms mediating immunosuppression, represent important unknowns in reproductive immunology. Like T cells, B cells possess the capacity to recognize specific fetal antigens, leading to their activation and expansion. B cell activation is strictly controlled to maintain balance between immunity and tolerance, which is especially important in the context of pregnancy and the antigenically foreign fetus. We are actively investigating a subset of B cells called regulatory B cells (Breg) that display powerful immune suppressive properties and can operate synergistically with, or independently of, Treg. Manipulation of these cells may lead to improved pregnancy outcomes, benefiting the health and lives of mothers and their babies.



