Providing iPSC Generation and Characterization Services to Researchers
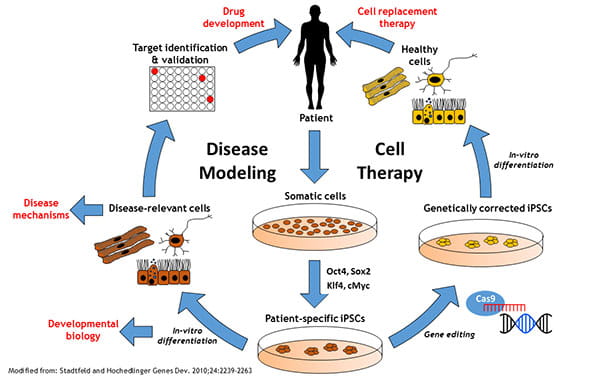
Initially described in the pioneering work of Yamanaka and colleagues, the ability to "reprogram" differentiated somatic cells into a pluripotent embryonic stem cell-like state by retroviral mediated expression of four specific transcription factors has revolutionized our ability to develop new models to study humans disease and represents a significant step toward patient-specific cell replacement therapies.
What can I do with iPSCs?
In addition to solving the ethical concerns related to the use of human blastocyst-derived embryonic stem cells, because it is possible to reprogram somatic cells derived from any individual, iPSC technologies provide a unprecedented tool for studying normal and pathological human development, and for developing novel models of human disease, including analysis of the effects of genetic variation on disease pathophysiology. In addition, iPSC-derived cells/tissues can be used for the discovery and validation of new therapeutic drug targets for treating human disease, and for identifying human-specific toxicities of drug candidates. Finally, the potential clinical application of cells/tissues derived from human iPSCs is also being evaluated in a number of clinical trials.