Projects
Projects
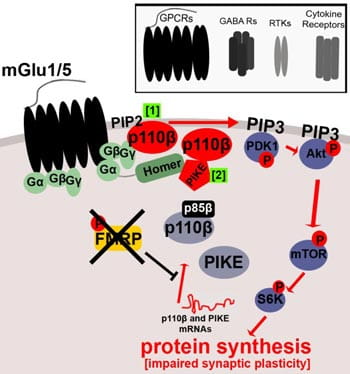
Our previous work focused on the molecular mechanisms underlying dysregulated synaptic protein synthesis, signal transduction and hyperexcitability in fragile X syndrome (FXS), an inherited intellectual disability and autism spectrum disorder. We have shown that the fragile X mental retardation protein (FMRP) regulates synaptic expression and enzymatic activity of the intracellular signaling complex, phosphoinositide-3 kinase (PI3K), leading to excess PI3K signaling in Fmr1 knockout mice (Gross et al., 2010). FMRP directly controls the mRNA translation and expression of at least two components of this pathway, the PI3K catalytic subunit p110β, and the PI3K enhancer PIKE, suggesting them as potential therapeutic targets for FXS and other autism spectrum disorders. In a subsequent study, we showed that excess and dysregulated protein synthesis in lymphoblastoid cell lines from patients with FXS could be rescued by a p110β subunit-selective inhibitor, pointing towards a novel therapeutic strategy for FXS (Gross and Bassell, 2012). We are currently following up on these findings using genetic and pharmacological methods.
FMRP directly regulates expression of the PI3K pathway components p110β (1) and PIKE (2), leading to enhanced and dysregulated PI3K signaling downstream of metabotropic glutamate receptors 1 and 5, but also of other G-protein coupled receptors (GPCRs), as well as GABA receptors, receptor tyrosine kinases (RTKs) and cytokine receptors.
Patients with fragile X syndrome have a higher susceptibility to epilepsy, but the exact mechanisms are unknown. Our previous work suggested a potential molecular mechanism by showing that FMRP controls the mRNA translation and cell surface expression of the A-type potassium channel Kv4.2, a major regulator of neuronal excitability in the hippocampus (Gross et al., 2011). The lab is currently following up on these findings by investigating how FMRP and microRNAs regulate Kv4.2 expression during epilepsy.
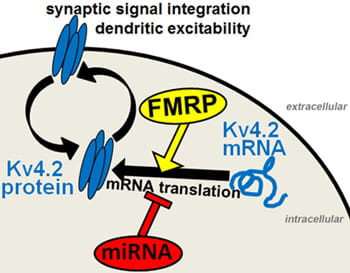
Proposed Model of Kv4.2 regulation: Expression levels of Kv4.2 in neurons are tightly controlled by FMRP and microRNAs that antagonize each other in regulating Kv4.2 mRNA translation. Increased Kv4.2 mRNA translation may counteract endocytosis of Kv4.2 induced by external stimuli. We hypothesize that defects in the regulation of Kv4.2 mRNA translation may contribute to epileptic seizures and epileptogenesis.
PI3K enzymatic activity is mediated by p110 catalytic subunits. There are four class 1 PI3K catalytic subunits, p110a, p110β, p110γ and p110δ, and recent research has shown that these subunits are not functionally redundant, but have specific roles downstream of distinct neuronal receptor signaling complexes. Moreover, the p110 subunits have been implicated in a subset of different neuronal diseases, including autism, epilepsy and schizophrenia. One focus of the Gross Lab is to understand the differential regulation and signaling of these catalytic subunits in neurons and how their dysfunction leads to disease.
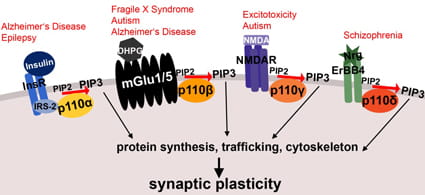
Taken from Gross C and Bassell GJ (2014) Front. Mol. Neurosci. 7:12. doi: 10.3389/fnmol.2014.00012.