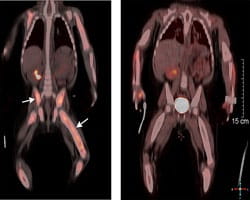
Recent Research in Histiocytic Disorders
Recent studies have shown that histiocytic disorders share the same genetic mutations as some cancers. These mutations include changes in the BRAF and MEK genes. Based on these findings, Cincinnati Children’s has started treating histiocytosis patients with oral medicines that block these gene mutations.
Since starting these treatments, Cincinnati Children’s has seen overwhelmingly positive results. Patients have responded to the treatment and have not relapsed while on treatment. With this success, our physician-scientists are actively pursuing clinical trials that study gene inhibitor treatment and compare them to the standard chemotherapy treatment.
There is one study currently in progress that is looking at the gene inhibitor trametinib and whether it is helpful in treating patients who have BRAF-V600 mutations and tumors that are recurrent or don’t respond to treatment. Approximately 50% to 60% of patients with Langerhans cell histiocytosis (LCH) have the BRAF-V600E mutation.
One of the trials we are pursuing will evaluate a new, MEK gene inhibitor as treatment for all patients with histiocytosis as frontline treatment. Recent findings have shown that MEK inhibitors may be an effective treatment for patients with a variety of genetic mutations, including those with BRAF mutations.
Our team is also conducting laboratory studies to determine how long certain patients need to use a treatment. The studies are looking at children with the BRAF-V600E gene mutation. Ultimately, we hope to learn how to predict when the mutant gene has completely disappeared from the body, so we can stop treatment.