Stephen N. Waggoner, PhD
- Associate Professor, UC Department of Pediatrics
About
Biography
As a postdoctoral trainee at the University of Massachusetts, I conducted work that led to the discovery of an immunoregulatory role of natural killer (NK) cells. This training — along with my interactions with friends suffering from autoimmune disease — fueled my current research interests.
At Cincinnati Children’s, I study viruses and diseases associated with viral infections, vaccines, autoimmune disease, inflammatory disease, innate immunity, immune regulation and cellular immunotherapy.
The goals of my research are three-fold:
- Improve and enable effective new vaccines for human disease
- Understand pathogenesis of infectious, autoimmune and allergic disease
- Develop new therapies capable of promoting sustained disease remission for lupus and other autoimmune conditions
Building on my early NK cell work, our lab has shown that NK cell regulatory activity constrains vaccine-induced immune responses and could be targeted to permit the development of effective vaccines. We have also been working on a cellular immunotherapy, which shows promise for the treatment of autoimmune disease.
I am a standing member of the National Institutes of Health HIV Immunopathogenesis and Vaccine Development Study Section. In 2014, I received the National Institute on Drug Abuse Avante-Garde Award for HIV/AIDS research. I have also received the Dr. Ralph and Marian Falk Medical Research Trust Catalyst Award.
BA: St. Mary's College of Maryland, St. Mary's City, MD, 2000,
PhD: University of Virginia, Charlottesville, VA, 2007,
Post Doc: University of Massachusetts Medical School, Worcester, MA,
Interests
Viral immunology; natural killer cells; immunoregulation; vaccines; autoimmunity; immune dysfunction in aging.
Research Areas
Publications
RNA helicase DDX3X promotes NK cell survival by supporting MCL1 expression. Journal of Immunology. 2025; 214(12):3218-3227.
Refined cell transfer model reveals roles for Ascl2 and Cxcr3 in splenic localization of mouse NK cells during virus infection. Journal of Immunology. 2025; 214(8):1917-1925.
Prothrombin prevents fatal T cell-dependent anemia during chronic virus infection of mice. JCI Insight. 2025; 10(4).
Progressive accumulation of hyperinflammatory NKG2Dlow NK cells in early childhood severe atopic dermatitis. Science immunology. 2024; 9(92):eadd3085.
Natural killer cell immunosuppressive function requires CXCR3-dependent redistribution within lymphoid tissues. Journal of Clinical Investigation. 2021; 131(18).
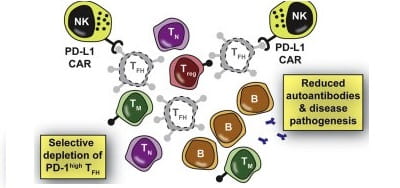
Therapeutic Targeting of Follicular T Cells with Chimeric Antigen Receptor-Expressing Natural Killer Cells. Cell Reports Medicine. 2020; 1(1).
IL-33 promotes type 1 cytokine expression via p38 MAPK in human NK cells. Journal of Leukocyte Biology. 2020; 107(4):663-671.
Dynamic Changes in Natural Killer Cell Subset Frequencies in the Absence of Cytomegalovirus Infection. Frontiers in Immunology. 2019; 10:2728.
Affinity Maturation Is Impaired by Natural Killer Cell Suppression of Germinal Centers. Cell reports. 2018; 24(13):3367-3373.e4.
Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells. Nature Communications. 2015; 6:6375.
From the Blog
Overactive Natural Killer Cells Linked to Asthma Progression
Stephen N. Waggoner, PhD, Gurjit Khurana Hershey, MD, PhD2/9/2024
Novel CAR NK-Cell Technology Could Lead to New Treatments for Lupus, Other Incurable Diseases
Stephen N. Waggoner, PhD, Hermine I. Brunner, MD, MSc, MBA5/11/2020