
Hermine I. Brunner, MD, MSc, MBA
- Director, Division of Rheumatology
- Professor, UC Department of Pediatrics
- hermine.brunner@cchmc.org
- Board Certified
About
Biography
As a clinical and translational scientist, I know I’m not alone in my desire to make a difference in people’s lives and find treatments that work. But, as a doctor and researcher focused on childhood rheumatic diseases, I feel especially motivated toward these goals. That’s because these conditions can be extremely painful and leave lasting, disabling effects on children.
Here at Cincinnati Children’s, I lead the Division of Rheumatology where we integrate clinical and translational research into daily care. My research work is made even more meaningful by interactions with the children and families I meet and serve in clinic, at our Lupus Center and within our lupus support groups.
I can trace my clinical and research interests back to a position I held during my medical training at Ludwig Maximillian University in Munich, Germany. There, I was part of a laboratory studying human leukocyte antigen (HLA) and its impact on transplanted tissue. I became fascinated with HLA’s role in regulating the immune system, and this early scientific experience set the tone for my career.
My research at Cincinnati Children’s is focused on the development of clinical trial endpoints, surrogate measures and biomarkers as they pertain to pediatric rheumatic diseases, particularly lupus. My colleagues and I conduct clinical trials in pediatric rheumatic disease, drug development, and biomarker discovery for lupus nephritis and lupus that affects the brain.
As part of my investigator-initiated research, I have successfully conducted several large multinational studies to develop flare, improvement, remission and inactive disease criteria for children with lupus.
I serve as the scientific director of the Pediatric Rheumatology Collaborative Study Group, where I work alongside researchers at more than 80 academic centers across the U.S. and Canada. As part of this network I design and conduct clinical trials to test new medications for the treatment of children with rheumatic diseases.
I find it fulfilling to be a part of many professional organizations related to pediatric rheumatic diseases and pediatric research. Our division is home to the Pediatric Rheumatology Clinical Outcome & Improvement Network (PCOIN), a multicenter learning network focused on connecting research and clinical care. My personal affiliations include the Childhood Arthritis and Rheumatology and Research Alliance, and the American College of Rheumatology.
I am committed to make our division excel in research, education, training and quality-driven patient-centered clinical care. This commitment has been recognized by such honors as the Castle Connolly Regional Top Doctor and Exceptional Women in Medicine awards (2017, 2018 and 2019), the Halsted R. Holman Award for Excellence in Clinical Research (2018) and the Above & Beyond Doctor of the Year award from the Aubrey Rose Foundation (2018).
MD: Ludwig Maximilan University, Munich, Germany, 1991,
Residency: University of Chicago, Chicago, IL, 1997,
Fellowship: University of Toronto & Cincinnati, 1999,
MSc: Clinical Epidemiology, University of Toronto, 1999,
Certifications: Pediatrics 1997; Rheumatology, 2002
Interests
Health-related quality of life; outcome research; lupus
Services and Specialties
Interests
Economic analyses; HRQOL; measurement development; outcome research; lupus
Research Areas
Additional Languages
German, PortugueseLocation
Insurance Information
Cincinnati Children's strives to accept a wide variety of health plans. Please contact your health insurance carrier to verify coverage for your specific benefit plan.
Publications
Pharmacokinetics and Safety of Ustekinumab in Patients with Juvenile Psoriatic Arthritis: Results of the Real-World Ustekinumab Pediatric Opportunistic Pharmacokinetics Study (U-POPS). Rheumatology and Therapy. 2026; 13(1):265-278.
The Renal Activity Index for Lupus: Validation for Prediction of Kidney Inflammation in Adult Patients With Lupus Nephritis. Journal of Rheumatology. 2026.
Characterization of TNF-Alpha Inhibitor Induced Paradoxical Psoriasiform Dermatitis at a Single Center via Retrospective Chart Review. Pediatric Dermatology. 2026; 43(1):84-87.
Juvenile Spondyloarthritis Disease Activity Index Validation in Enthesitis-Related Arthritis and Juvenile Psoriatic Arthritis in a Prospective Clinical Trial Setting. Journal of Rheumatology. 2026; 53(1):85-94.
Cognitive Behavioral Therapy for Youth with Childhood-Onset Lupus: A Randomized Clinical Trial. Arthritis Care and Research. 2025.
Validation of the Pediatric Arthritis Ultrasound Scoring System for the Elbow, Wrist, and Finger Joints in Children With Juvenile Idiopathic Arthritis. Arthritis Care and Research. 2025.
Pharmacokinetics, Pharmacodynamics, and Safety of Subcutaneous Belimumab in Pediatric Patients with Systemic Lupus Erythematosus: A Multicenter, Open-Label Trial. Arthritis Care and Research. 2025.
2025 American College of Rheumatology (ACR) Guideline for the Treatment of Systemic Lupus Erythematosus. Arthritis & Rheumatology. 2025.
2025 American College of Rheumatology (ACR) Guideline for the Treatment of Systemic Lupus Erythematosus. Arthritis Care and Research. 2025.
Navigating the Biosimilars from Bench to Bedside in Juvenile Idiopathic Arthritis. Paediatric Drugs. 2025; 27(6):679-691.
From the Blog
NIAMS-Funded Clinical Trial Featured on Lupus Foundation Podcast
Hermine I. Brunner, MD, MSc, MBA, Prasad Devarajan, MD, FAAP, FASN2/9/2024
Tofacitinib Receives FDA Approval for Children with pcJIA
Hermine I. Brunner, MD, MSc, MBA12/10/2020
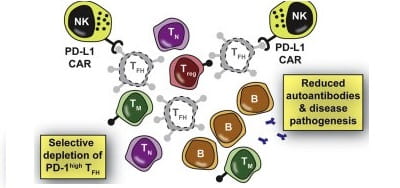
Novel CAR NK-Cell Technology Could Lead to New Treatments for Lupus, Other Incurable Diseases
Hermine I. Brunner, MD, MSc, MBA, Stephen N. Waggoner, PhD5/11/2020






Patient Ratings and Comments
All patient satisfaction ratings and comments are submitted by actual patients and verified by a leading independent experience management company, Qualtrics. Patient identities are withheld to ensure confidentiality and privacy. Only those providers whose satisfaction surveys are administered through Cincinnati Children’s Hospital Medical Center are displayed. Click here to learn more about our survey