Generating Human Tissues from Pluripotent Stem Cells
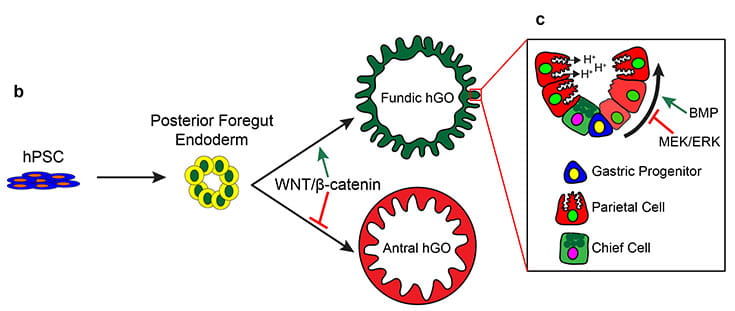
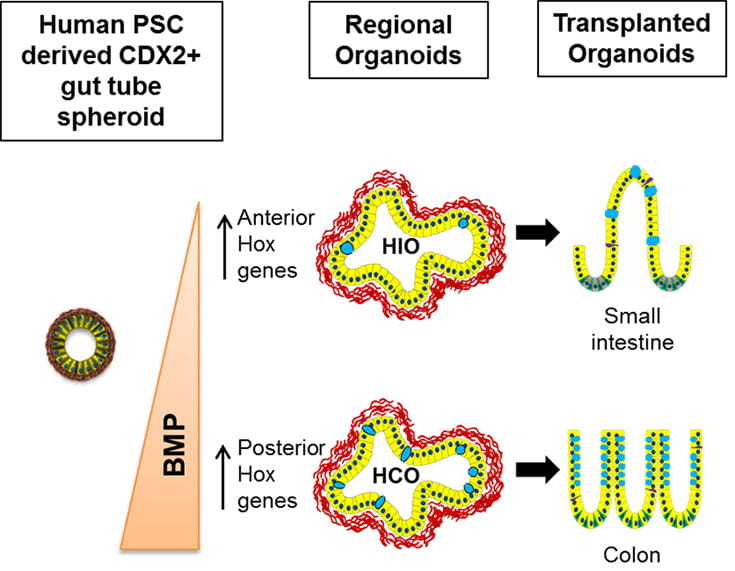
Using the signaling pathways that control organ development in vivo, we can direct the in vitro differentiation of pluripotent stem cells into human organ tissues called organoids. Organoids can be used to study human organ development or can be used to study congenital and infectious diseases. Examples include gastrointestinal (GI) tissues (esophagus, stomach, intestine, colon) that are used to study malabsorption syndromes and infectious diseases of the GI tract and endocrine cells, including pancreatic beta cells that we use to study genetic forms of diabetes.
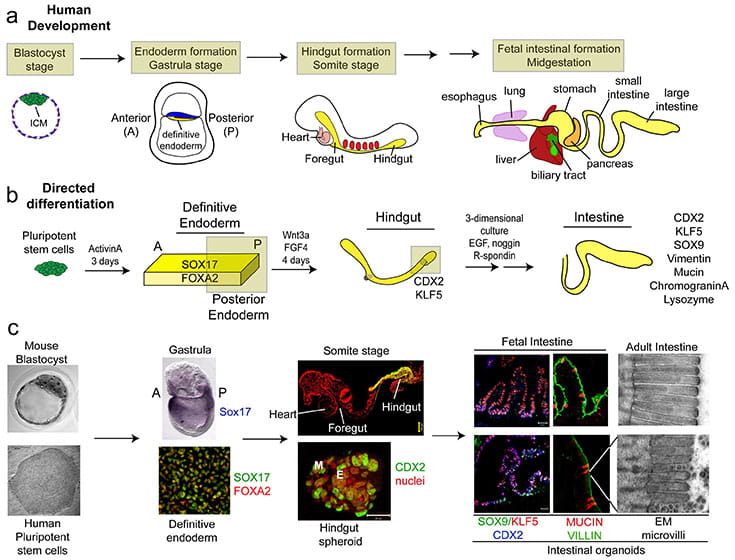
Differentiation of Human PSCs into Intestinal Tissue (Spence et al., 2011. Nature)
Studies in embryonic development have guided successful efforts to direct the differentiation of human embryonic and induced pluripotent stem cells (PSCs) into specific organ cell types in vitro. For example, human PSCs have been differentiated into monolayer cultures of liver hepatocytes and pancreatic endocrine cells that have therapeutic efficacy in animal models of liver disease and diabetes respectively. However the generation of complex three-dimensional organ tissues in vitro remains a major challenge for translational studies. We have established a robust and efficient process to direct the differentiation of human PSCs into intestinal tissue in vitro using a temporal series of growth factor manipulations to mimic embryonic intestinal development. This involved activin-induced definitive endoderm (DE) formation; FGF/Wnt induced posterior endoderm pattering, hindgut specification and morphogenesis; and a pro-intestinal culture system to promote intestinal growth, morphogenesis and cytodifferentiation. The resulting three-dimensional intestinal “organoids” consisted of a polarized, columnar epithelium that was patterned into villus-like structures and crypt-like proliferative zones that expressed intestinal stem cell markers. The epithelium contained functional enterocytes, as well as goblet, Paneth, and enteroendocrine cells. Using this culture system as a model to study human intestinal development, we identified that the combined activity of Wnt3a and FGF4 is required for hindgut specification whereas FGF4 alone is sufficient to promote hindgut morphogenesis. Our data suggests that human intestinal stem cells form de novo during development. Lastly we determined that NEUROG3, a pro-endocrine transcription factor that is mutated in enteric anendocrinosis, is both necessary and sufficient for human enteroendocrine cell development in vitro. In conclusion, PSC-derived human intestinal tissue should allow for unprecedented studies of human intestinal development and disease.