Organ Function and Dysfunction: Diabetes and Digestive Diseases
The Wells Lab uses pluripotent stem cells to to study patients with genetic forms of diabetes and digestive diseases.
Generating Human Intestinal Tissue with a Functional ENS to Study Hirschsprung’s Disease (Workman and Mahe et al., 2017. Nat Med)
 The enteric nervous system (ENS) of the gastrointestinal (GI) tract controls many diverse functions including motility and epithelial permeability. Perturbations in ENS development or function are common, yet a human model to study ENS-intestinal biology and disease is lacking.
The enteric nervous system (ENS) of the gastrointestinal (GI) tract controls many diverse functions including motility and epithelial permeability. Perturbations in ENS development or function are common, yet a human model to study ENS-intestinal biology and disease is lacking.
We have used a tissue engineering approach with pluripotent stem cells (PSCs) to generate human intestinal tissue containing a functional ENS. We recapitulated normal intestinal ENS development by combining PSC-derived neural crest cells (NCCs) with developing human intestinal organoids (HIOs). NCCs recombined with HIOs in vitro migrated into the mesenchyme, differentiated into neurons and glial cells, and had neuronal activity as measured by rhythmic waves of calcium transients. ENS-containing HIOs grown in vivo formed neuroglial structures similar to a myenteric and submucosal plexus, had functional interstitial cells of Cajal, and had an electro-mechanical coupling that regulated waves of propagating contraction. Lastly, we used this system to investigate the cellular and molecular basis for Hirschprung’s disease caused by a mutation in the gene PHOX2B. This is the first demonstration of a human PSC-derived intestinal tissue with a functional ENS and how this system can be used to study motility disorders of the human gastrointestinal tract.
Genetic Forms of Diabetes in Humans
We are using PSC models to study patients with genetic forms of diabetes to both identify the molecular basis of the disease and to explore new genetic and cell based therapies to cure diabetes.
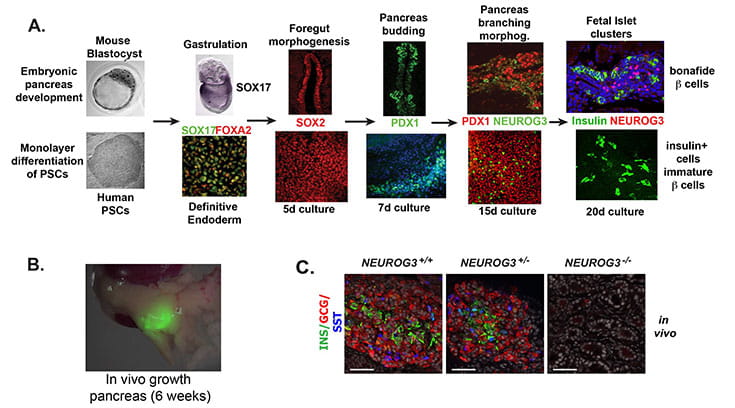
NEUROGENIN3 is required for human endocrine pancreas development (from McGrath et al., Diabetes. 2015).
Neurogenin 3 (NEUROG3) is a basic helix-loop-helix transcription factor that is required for development of the endocrine pancreas in mice. In contrast, humans with NEUROG3 mutations are born with endocrine pancreas function, calling into question whether NEUROG3 is required for human endocrine pancreas development. To test this directly, we generated human embryonic stem cell (hESC) lines where both alleles of NEUROG3 were disrupted using CRISPR/Cas9-mediated gene targeting. NEUROG3-/- hESC lines efficiently formed pancreatic progenitors, but lacked detectible NEUROG3 protein and did not form any endocrine cells in vitro. Moreover, NEUROG3-/- hESC lines were unable to form mature pancreatic endocrine cells following engraftment of PDX1+/NKX6.1+ pancreatic progenitors into mice. In contrast, a 75-90% knockdown of NEUROG3 caused a reduction, but not loss, of pancreatic endocrine cell development. We conclude that NEUROG3 is essential for endocrine pancreas development in humans and that as little as 10% NEUROG3 is sufficient for formation of pancreatic endocrine cells.