Current Projects
Current Projects
Preterm labor and delivery are a frequent, and often devastating, complication of pregnancy that have remained refractory to medical intervention. The limited ability to treat preterm labor chiefly stems from inadequate understanding of the mechanisms controlling the normal onset of labor, and the potential ways in which these mechanisms can malfunction. To define the mechanism of parturition control, we have utilized genetic methods in both mice and humans.
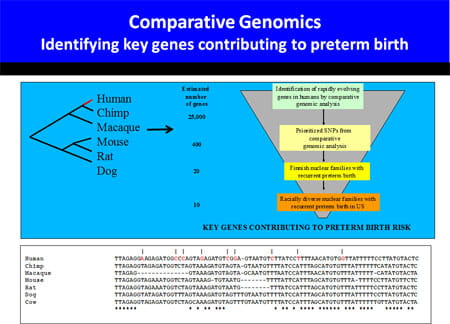
Our initial parturition studies systematically determined the importance of oxytocin (OT) and prostaglandins (PG) for term labor, two pathways that had been shown by pharmacological means to promote labor (Gross et al. PNAS 1998). Utilizing microarray approaches we have compared murine and human gestation (Bethin et al. Mol Endo 2003).
These studies implicate little overall conservation of gene regulatory changes in the uterus of mice and humans as labor approaches. However, the gene expression signature of human term and preterm labor appear quite similar. This finding suggests that spontaneous idiopathic preterm labor may represent a shift in the clock timing gestation, activating the normal parturition cascade prematurely.
To gain greater insight into human birth, we have embarked on a large effort to identify families with multiple episodes of prematurity for linkage and association studies. Candidate genes will be developed by a novel comparative genomic approach.
Our laboratory seeks to elucidate the molecular pathways involved in the behavioral and neuroendocrine responses to stress, and to determine how these mechanisms affect development and aging.
Importantly, chronic stress often results in alterations in behavior and physiology that are maladaptive, exacerbating both medical and psychiatric diseases. Our laboratory has analyzed mice with corticotropin-releasing hormone (CRH) deficiency to determine the role of this hypothalamic neuropeptide in adrenal axis regulation and fetal development.
These studies demonstrated a critical role for CRH in promoting glucocorticoid secretion during metabolic and psychological stressors, and for glucocorticoids in promoting normal lung development.
To definitively determine the importance of glucocorticoids on specific immune cell populations and in the brain, we have generated mice with conventional and conditional tissue-specific inactivation of the glucocorticoid receptor (Brewer et al. Nat Med 2003; Boyle et al. Proc Natl Acad Sci USA 2005; Bhattacharyya et al. Blood 2007). These studies have revealed an essential role for the glucocorticoid receptor to limit inflammatory responses after immune cell activation, and modify anxiety, learning and memory.
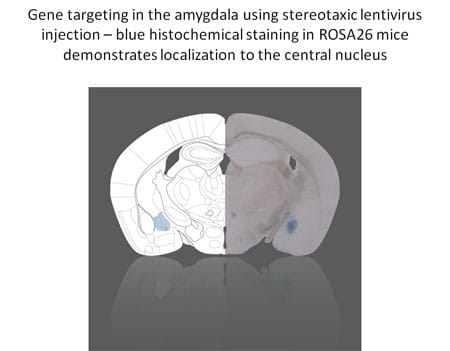
The calcium-stimulated adenylyl cyclases (ACs) provide a critical control point for the regulation of neuronal physiology, and have been implicated in activity-dependent alterations in neural function. To further elucidate the mechanisms involved in the behavioral and adrenal responses to chronic stress, we have generated mice deficient in adenylyl cyclase type VIII (AC8), the only calcium-stimulated adenylyl cyclase expressed in the hypothalamus.
These mice demonstrate significant abnormalities in adaptation to chronic stress, and serve as a valuable model of stress-related learning. The ramifications of loss of calcium-stimulated adenylyl cyclase activity extend beyond modulation of the stress response.
We have additionally demonstrated substantial defects in synaptic plasticity, long-term potentiation, and learning and memory in mice deficient in both AC1 and AC8, and thus all calcium-stimulated AC activity (Wong et al. Neuron 1999).
Based upon the interaction of NMDA-receptor signaling and activation of AC1 and AC8, we have recently demonstrated significantly enhanced sensitivity of mice deficient in these AC isoforms to the neurotoxic effects of ethanol during brain development (Maas et al. J Neurosci 2005). This finding will shed light on the mechanisms of damage occurring in fetal alcohol syndrome, a common, important pediatric disease.
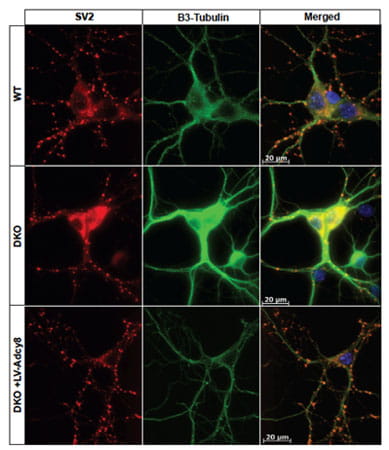
Adenylyl cyclase type 8 regulates SV2 expression. Shown are representative images of SV2 distribution in cultured hippocampal neurons. SV2 is labeled with CY3 (red). β3-tubulin, a neuronal marker, is labeled with Alexa 488. Dapi is used to visualize nuclei. SV2 is reduced in DKO cultures, but not DKO cultures infected with Lentivirus-Adcy8



