Translational Research
Our translational studies focus on uncovering biomarkers for macrophage activation syndrome (MAS) and understanding the relation of genetic differences in MAS patients to cell function. Our studies also examine the use of a novel imaging agent to assess disease in mouse models of arthritis.
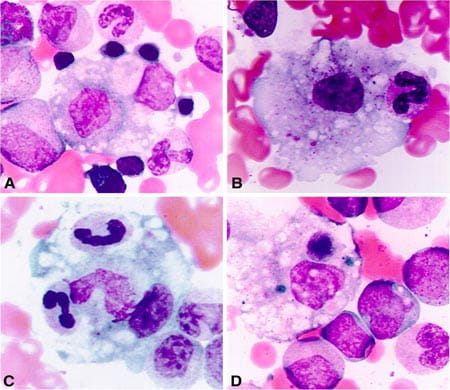
Systemic Juvenile Idiopathic Arthritis and an associated condition known as Macrophage Activation Syndrome are severe and often devastating illnesses. In fact, MAS still remains one of the major causes of morbidity and mortality in pediatric rheumatology. There are no validated diagnostic for MAS, and in clinical practice, there is a strong need for biomarkers that would help identify SJIA patients at risk for the development of MAS as well as reliable laboratory tests that would assist with the early diagnosis of MAS. These studies, however, are complicated by the absence of a reliable animal model of systemic JIA. Based on strong clinical similarities between MAS and better understood familial hemophagocytic lymphohistiocytosis (FHLH), a constellation of autosomal recessive disorders all linked to various genetic defects affecting the granule-dependent perforin-mediated cytolytic activity, Dr. Grom’s research has focused on the clinical, pathophysiologic, and genetic overlap between systemic JIA, MAS, and FHLH.
Whole exome sequencing in MAS/SJIA trios (funded through collaboration with Novartis Institute for Biotechnology, Basel, Switzerland) and deep sequencing of the MAS associated MUNC13-4 region (funded by NIH R01-AR059049) identified several candidate genes likely to be involved in MAS/HLH (manuscript in preparation). The role of these genes will be further explored in functional mechanistic studies. Identification of transcriptional mRNA and miRNA changes during macrophage differentiation in MAS funded through PO1-AR048929 (Project #4) is expected to identify new diagnostic biomarkers. Translational studies will be designed to explore the clinical utility of these biomarkers. Translational and epidemiologic studies focused on the effects of new biologics on the incidence and clinical presentation of MAS in SJIA are performed in collaboration with Novartis Institute for Biotechnology (Basel, Switzerland). Identification of new therapeutic targets in MAS is the main goal of preclinical studies performed in collaboration with NovImmune (Geneva, Switzerland).
Genomics of Reactive Hemophagocytic Lymphohistiocytosis
This project identifies novel genetic factors that impact the risk for development of reactive HLH in children with severe pulmonary diseases, including influenza infection and systemic juvenile idiopathic arthritis.
Support: NIH K12-HL119986
Contribution of microRNA to aberrant monocyte activation and polarization in systemic juvenile idiopathic arthritis
These studies provide the first examination of miRNA expression profiles in patients with SJIA. This emerging area has the potential to reshape our understanding of the molecular mechanisms controlling macrophage activation and differentiation. The long term goal is to enhance the understanding of cellular mechanisms underlying innate immune dysfunction leading to rheumatic disease in children.
Support: Rheumatology Research Foundation
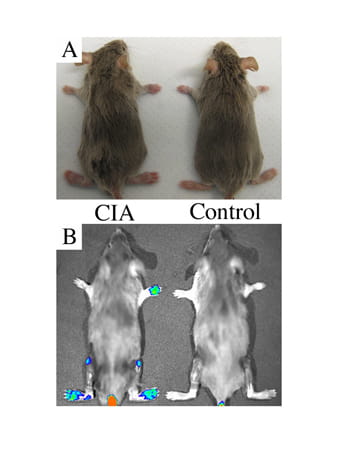
SapC-DOPS Agents: Imaging and Therapeutics in Arthritis
SapC-DOPS is a reagent that targets stressed cells. We are using it to determine if, through the addition of a fluorophore, we can image arthritis earlier in animals than can be done with macroscopic scoring. This novel technology provides the sensitivity required to assess arthritic disease onset and progression in live subjects. This line of research may eventually provide a means for targeted local delivery of therapeutic biologics. These studies are being done in conjunction with Xiaoyang Qi, PhD, (UC Cancer Biology).