Chandrashekhar Pasare, DVM, PhD
- Professor, UC Department of Pediatrics
About
Biography
In my research, I pursue topics such as regulation of inflammation, dendritic cell biology, innate immunity, toll-like receptor signaling and the cross-talk between the innate and adaptive immune systems. Our lab’s primary goals are to understand how the innate and adaptive immune system interact with each other and to define the molecules that are part of this interaction.
We are also studying how inflammation is prompted by microbial recognition and during autoimmunity. In general, our objectives are 1) to obtain a molecular understanding of inflammation to develop new treatments for reducing autoimmunity and inflammation and 2) to define the complex communication between innate and adaptive immune systems in order to improve the design of vaccines.
Some of the most notable discoveries my colleagues and I have made include:
- Discovering a completely new pathway for inflammation in which auto-reactive T cells guide the innate immune system to produce IL-1beta; the new pathway may be used to treat auto-immunity
- Finding a new Toll-Like Receptor signaling adapter known as BCAP that reduces inflammation
My lab concentrates on identifying the innate immune system's main activation mechanisms and its effect on inflammation and adaptive immunity. The innate immune system depends on certain receptors known as pattern recognition receptors to distinguish pathogens.
Pathogen recognition via the innate immune system causes inflammation as well as adaptive immunity activation. One of my lab's major objectives is to identify the elaborate interaction and signaling between the innate and adaptive immune systems. My colleagues and I are specifically pursuing how the innate immune system prompts inflammation and how it affects both protective immunity and inflammatory conditions.
My research interests began due to my fascination with learning how the body’s immune system knows not to react to self but recognizes and responds to the microbial non-self. Our research has defined multiple new proteins and pathways in cells within innate and adaptive immune systems. We have utilized them as handles to direct questions regarding protective immunity, cancer and inflammatory diseases. We employ cutting-edge in vitro and in vivo methods in our research that may bring new targets to treat auto-immunity, cancer and inflammatory conditions.
I have more than twenty years of experience in the field of immunology and first began working at the Cincinnati Children’s Hospital Medical Center in 2018. My research has been published in numerous journals, including Nature, Science, Immunity, The Journal of Experimental Medicine, Nature Immunology, Cell Reports, Journal of Immunology and Nature Communications.
PhD: National Institute of Immunology, New Delhi, India, 2000,
Fellowship: Post Doctoral, Immunobiology, Yale University School of Medicine, New Haven, CT, 2006,
Interests
Innate immunity; toll-like receptor signaling; dendritic cell biology; innate control of adaptive immunity; regulation of inflammation
Research Areas
Publications
Absence of BCAP in myeloid cells abrogates M2 macrophage differentiation and promotes anti-tumor immunity. iScience. 2025; 28(11):113723.
Memory CD8+ T cell-induced activation of the innate immune system drives inflammation and anti-viral immunity 3109. Journal of Immunology. 2025; 214(Supplement_1).
Immune checkpoint blockade induces de novo CD8 T cell priming and innate inflammation leading to myocarditis 3714. Journal of Immunology. 2025; 214(Supplement_1).
Bidirectional Communication Between the Innate and Adaptive Immune Systems. Annual Review of Immunology. 2025; 43(1):489-514.
B cell adapter for PI 3-kinase (BCAP) coordinates antigen internalization and trafficking through the B cell receptor. Science Advances. 2024; 10(46):eadp1747.
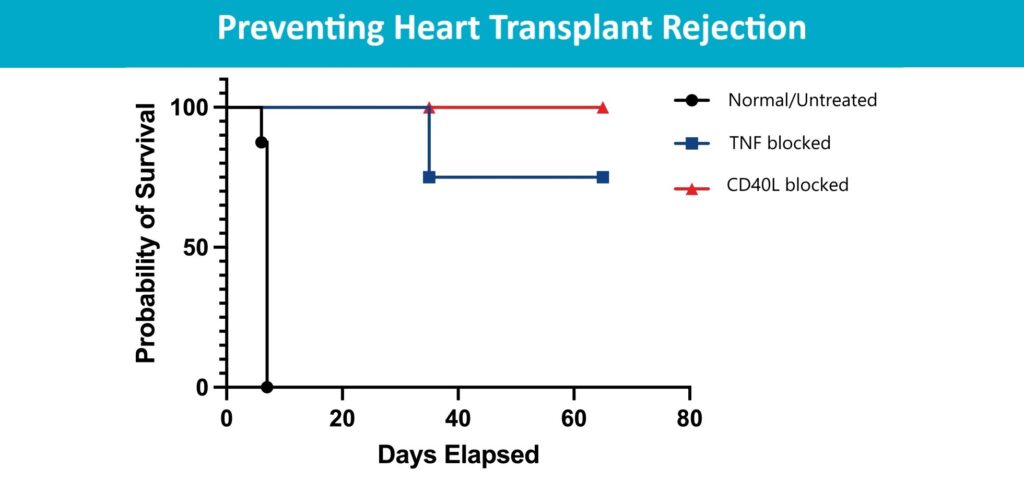
Alloreactive memory CD4 T cells promote transplant rejection by engaging DCs to induce innate inflammation and CD8 T cell priming. Proceedings of the National Academy of Sciences of the United States of America. 2024; 121(34):e2401658121.
Control of adaptive immunity by pattern recognition receptors. Immunity. 2024; 57(4):632-648.
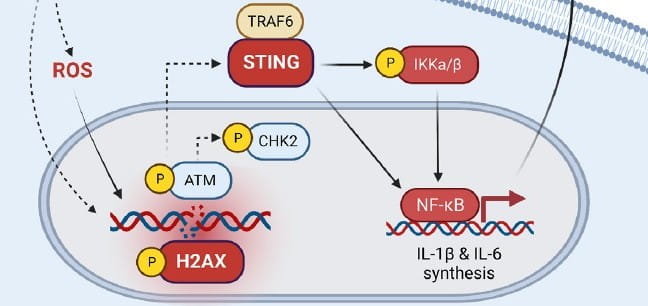
Effector memory T cells induce innate inflammation by triggering DNA damage and a non-canonical STING pathway in dendritic cells. Cell reports. 2023; 42(10):113180.
70 C5a receptor 1 controls antigen and TLR-driven T cell proliferation and differentiation by splenic conventional type 2 dendritic cells. Immunobiology. 2023; 228(5):152521.
IEC-intrinsic IL-1R signaling holds dual roles in regulating intestinal homeostasis and inflammation. Journal of Experimental Medicine (JEM). 2023; 220(6).
From the Blog
Discovery Could Make Immune Checkpoint Inhibitors Safer
Chandrashekhar Pasare, DVM, PhD, Jeffery D. Molkentin, PhD2/20/2026
Pre-Surgical Antibody Treatment Might Prevent Heart Transplant Rejection
Chandrashekhar Pasare, DVM, PhD8/12/2024
Can Science Take the STING Out of Runaway Inflammation?
Chandrashekhar Pasare, DVM, PhD10/3/2023
New Leader for Immunobiology: Chandrashekhar Pasare, DVM, PhD
Chandrashekhar Pasare, DVM, PhD9/7/2023
Study to Decode Microbe-Gut Signaling Suggests Potential New Treatment For IBD
Chandrashekhar Pasare, DVM, PhD4/3/2023
Targeting RipIL-33 Pathway Could Transform Allergy Treatment
Chandrashekhar Pasare, DVM, PhD, Marc E. Rothenberg, MD, PhD2/8/2023