Learn About Our Cohorts
Dr. Khurana Hershey has built and contributed to several innovative longitudinal cohorts over the last 25 years, which provide a tremendous infrastructure for research into the pathogenesis of allergic disorders and enable epidemiologic and mechanistic studies. These cohorts include the Greater Cincinnati Pediatric Clinic Repository (GCPCR), the Ohio Pediatric Asthma Repository (OPAR), the Mechanisms of Progression of Atopic Dermatitis to Asthma in Children (MPAACH), and the Cincinnati Childhood Asthma and Air Pollution Study (CCAAPS) birth cohort. These cohorts have resulted in numerous publications and critical new insights into the natural history, epidemiology, and mechanistic underpinnings of allergic diseases. These cohorts provide a tremendous and unique infrastructure for translation of findings from epidemiologic studies to the laboratory and back to human patients.
Read some of our related publications.
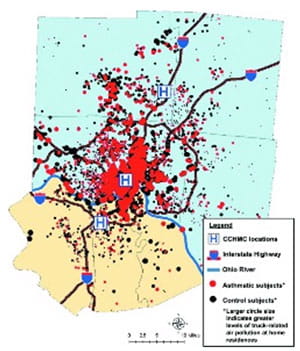 Greater Cincinnati Pediatric Clinic Repository (GCPCR)
Greater Cincinnati Pediatric Clinic Repository (GCPCR)
The Greater Cincinnati Pediatric Clinic Repository (GCPCR) was initiated in 1998 by Dr. Khurana Hershey to link and efficiently utilize clinical and epidemiologic data and biospecimens in order to delineate phenotypes of asthma and other allergic disorders (Pediatr Allergy Immunol Pulmonol 2012; 25:104-13). Children are invited to participate in the GCPCR upon presentation to a Cincinnati Children's Hospital Medical Center clinic or ED. At the time of recruitment, patients also are asked to provide a DNA sample. GCPCR includes over 7,000 participants with asthma, food allergy, and / or allergic rhinitis, as well as non-asthmatic, non-allergic controls (J Allergy Clin Immunol. 2017 Aug;140(2):595-598.e5. doi: 10.1016/j.jaci.2017.02.003). DNA samples from over 1,700 participants have undergone genome wide SNP genotyping. The genetic data collected as a part of this resource have contributed to numerous peer-reviewed manuscripts and grants. This repository is a rich resource and serves as the basis of several projects, including funded projects for several faculty, as well as providing a foundation for recruitment for clinical research studies.
 Mechanisms of Progression of Atopic Dermatitis to Asthma in Children (M-PAACH)
Mechanisms of Progression of Atopic Dermatitis to Asthma in Children (M-PAACH)
The Mechanisms of Progression of Atopic Dermatitis (AD) to Asthma in CHildren (MPAACH) cohort, the first US prospective longitudinal early life cohort of AD, was designed to meet the stated need a new early life longitudinal cohort to define phenotypic/endotypic subgroups of AD and predict AD outcomes. It is the cornerstone of our NIH U19 Asthma and Allergic Diseases Cooperative Research Center. As part of MPAACH, children complete comprehensive annual visits - longitudinal clinical and molecular data collection (omics, biome, immunophenotyping, genetics), as well as extensive longitudinal biospecimen collections and objective quantified environmental exposure data. MPAACH visits are ongoing and the cohort currently includes 700 children. MPAACH includes 65% Black children and is one of the only early life cohorts that represents this historically underrepresented and understudied population.
 Ohio Pediatric Asthma Repository (OPAR)
Ohio Pediatric Asthma Repository (OPAR)
The Ohio Pediatric Asthma Repository (OPAR) was initiated and funded by the state of Ohio in an effort to better understand the childhood asthma epidemic in the state of Ohio, where one in six children has asthma. The central objective of the Ohio Pediatric Asthma Repository (OPAR) is to build a comprehensive biorepository linking biosamples with clinical, demographic, adherence, health literacy, environmental, and health outcomes data, which provides an infrastructure for studies aimed at delineating asthma phenotypes and endotypes, and to determine the care practices across the participating Ohio children’s hospitals that were associated with the most optimal outcomes. A secondary objective was to provide a resource to stimulate collaborative research in asthma. The six participating Ohio Children’s Hospitals are Cincinnati Children’s Hospital Medical Center, Nationwide Children’s Hospital, Dayton Children’s Hospital, Akron Children’s Hospital, Rainbow Babies and Children’s Hospital, and ProMedica Toledo Children’s Hospital.
 Cincinnati Childhood Allergy and Air Pollution Study Birth Cohort (CCAAPS)
Cincinnati Childhood Allergy and Air Pollution Study Birth Cohort (CCAAPS)
The Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) is a birth cohort of 762 children born to at least one parent with allergy, which was originally funded by the NIEHS. Children were identified from birth records in a seven-county area of the Greater Cincinnati Area between 2001 and 2003. The children were examined for the presence of allergic disease annually at ages one to four and again at ages seven and 12 where asthma was objectively diagnosed. DNA and hair samples were collected at each research visit and nicotine and cotinine has been quantified in the hair samples from ages two, four and seven. All children’s DNA samples have undergone genome wide SNP genotyping. The data collected as part of CCAAPS has contributed to over 100 peer-reviewed publications. CCAAPS is a rich resource and provides the infrastructure for several collaborative projects, including as part of the ECHO-CREW national network.
 Inner-City Asthma Consortium (ICAC)
Inner-City Asthma Consortium (ICAC)
Inner city asthma initiative, funded by the National Institute of Allergy and Infectious Diseases (NIAID). Nationwide clinical trials network to evaluate new therapies and management strategies, to reduce the burden of asthma in children and adolescents living in the inner city.
 Children’s Repository and Environmental Workgroup-Phase II (ECHO CREW02-CCAAPS)
Children’s Repository and Environmental Workgroup-Phase II (ECHO CREW02-CCAAPS)
A phase II extension study from CCAAPS. It is one of twelve cohorts in CREW across the country looking for the relationship between childhood asthma and early-life environmental factors that cause childhood asthma.



