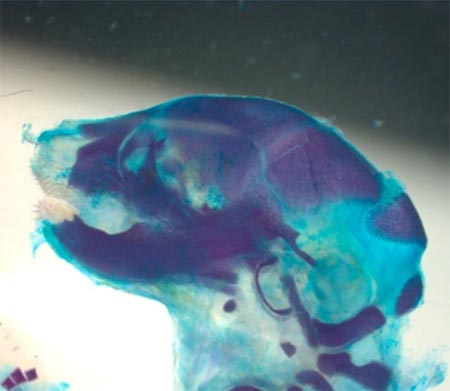
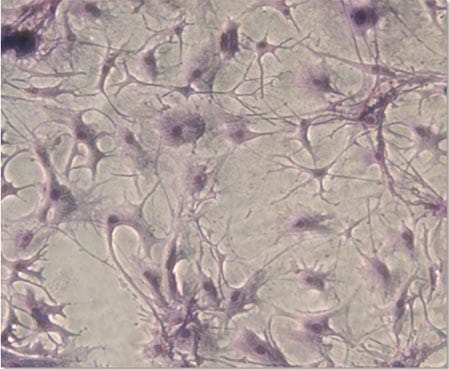
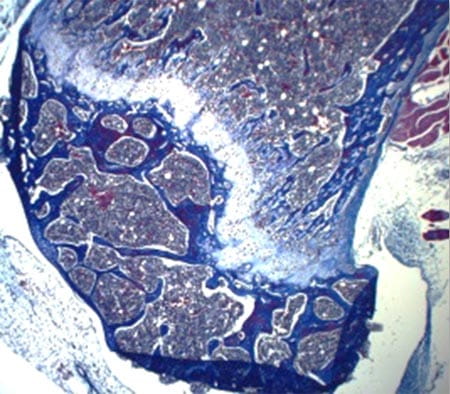
Mechanism of HSC self-renewal by the stem cell niche
Hematopoiesis takes place in specialized bone marrow microenvironment, called ‘niche’. The stem cell niche is composed of several cell types that produce regulatory cytokines and offer physical support that are essential for HSC localization, self-renewal and differentiation.
The fundamental issue in stem cell biology that has yet to be understood is how the hematopoietic stem cell niche is generated during embryogenesis and how it controls HSC functions at a molecular level. Mesenchymal stem cells are multipotent stem cells that are thought to give rise to several cell types that constitute the HSC niche, including osteoblasts, stromal cells and adipocytes. However, how they do so in vivo and whether this is important for the development of the hematopoietic system is still largely unknown.
The lab is interested in understanding how signaling pathway controls MSC fate and development into a functional stem cell niche. We have recently discovered that the Rho GTPase regulator p190-B RhoGAP is also a critical regulator of mesenchymal stem cell fate differentiation (at least to chondrocytes, osteoblasts and adipocytes) and functions that are necessary for hematopoietic tissue homeostasis. The objectives of this program research are to investigate how p190-B regulates bone marrow niche development and to identify the signal pathways that are responsible for its effect and how this affects hematopoietic development.