Signaling pathways in hematopoietic stem cell fate decisions
Multipotent hematopoietic stem cells (HSC) can both perpetuate themselves (self-renew) and give rise to progenitor cells that differentiate into all mature blood cells, in order to maintain hematopoiesis throughout life. The differentiation of HSCs into subsequent progenitors is associated with loss of their self-renewal potential, the progressive restriction in their lineage potential, and at the same time the acquisition of specific lineage features.
One fundamental focus of stem cell biology research is the drive to understand how HSC self-renew and how they make the choice to generate another stem cell or a progenitor cell in order to produce an adequate number of both HSC and mature cells – and, thus, to maintain tissue homeostasis and to reduce the potential of cell overgrowth and cancer/leukemia development.
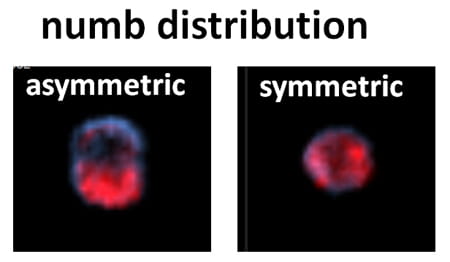
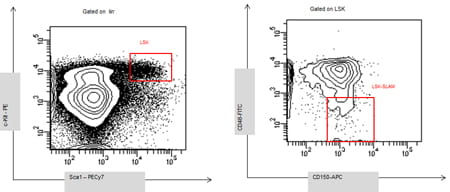
HSCs decisions are controlled by environmental cues. The lab investigates how signaling pathways integrate environmental cues into transcriptional responses that impart their fate. Our laboratory has identified a novel regulatory pathway of HSC self-renewal. We have shown that p190-B RhoGAP, a negative regulator of Rho GTPase activity, is a crucial regulator of HSC self-renewal by controlling HSC fate decisions during divisions, so-called, asymmetric versus symmetric self-renewal divisions. The current goals of this program research are to dissect in detail how p190-B controls asymmetric self-renewal divisions, and identify the signaling pathways that are important for this process. To do so, we are using candidate gene approaches, microarray and chemical screen analyses. We also investigate how p190-B-mediated cell polarization contributes to asymmetric self-renewal divisions. We are using cell imaging (standard immunofluorescence and image/flow analysis) and single cell multipotent lineage differentiation analysis. Finally, we want to identify how signaling pathways modulates HSC fate at a molecular level focusing on their role on regulating epigenetic programs.
These studies have tremendous implications for clinical HSC transplantation and for our understanding of fundamental mechanisms of normal and abnormal self-renewal processes that may lead to cancer development.