Current Projects
We pursue several projects elucidating the disease mechanisms of liver autoimmunity and testing out novel therapeutics in preclinical models. Also, we conduct studies to identify and validate biomarkers which will inform clinical management and may serve as surrogate endpoints in future clinical trials. The team is pursuing translational and clinical research through the Center for Autoimmune Liver Disease (CALD) in Cincinnati and through the Autoimmune Liver Network for Kids (A-LiNK) and the Childhood Liver Disease Research Network (ChiLDReN) across the US and Canada. The laboratory serves as a place to train future clinician investigators in pediatric hepatology.
Disease Mechanisms
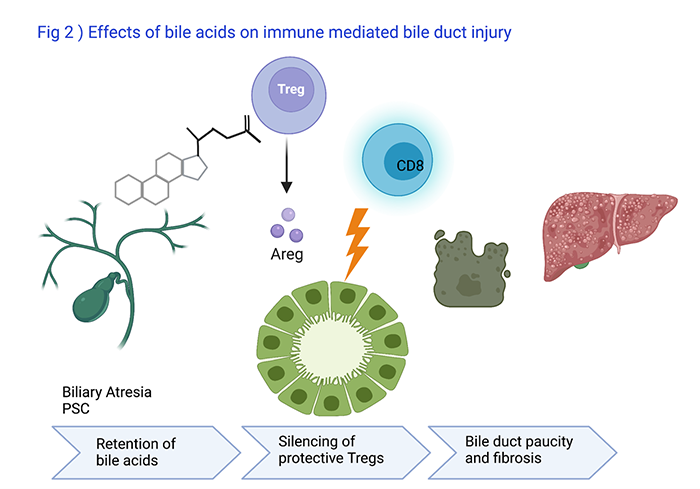
Bile Acids effects on Immune Mediated Bile Duct Injury
Ramesh Kudira, PhD, Research Associate, leads a program to understand how bile acids which are retained in the liver in patients with biliary atresia or PSC influence the functions of immune cells (Fig 2). So far, he has found out that hydrophobic bile acids silence protective regulatory T cells (Treg) and reduce production of Amphiregulin (Areg) which unleashes cytotoxic CD8 lymphocytes injuring bile duct epithelial cells in animal models. He presented the findings at the 2021 AASLD Annual Meeting.
Funding & Acknowledgements:
NIH/NIDDK
- R01 DK095001: Control of hepatic T cell responses in biliary atresia
- U01 DK62497: Clinical center for cholestatic liver disease in children
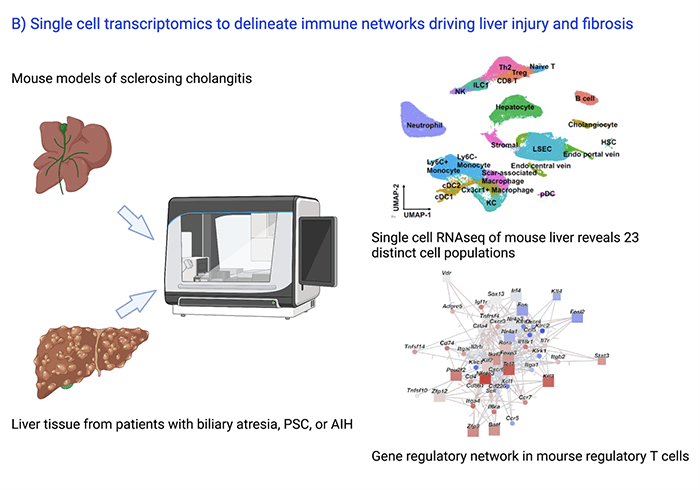
Discovery of Gene Regulatory Networks Driving Liver Injury and Fibrosis
In collaboration with Emily Miraldi, PhD, from the Divisions of Immunobiology and Biomedical Informatics at Cincinnati Children’s, Ramesh Kudira, PhD, and Zi Yang, PhD, student at the Molecular Developmental Biology Program, perform single cell RNAseq and ATAC seq experiments to decipher the gene regulatory and cell-cell communication networks controlling liver injury and fibrosis in cholestatic and inflammatory liver diseases (Fig 3). Preliminary findings were presented at the 2021 AASLD Annual Meeting. In collaboration with Lee Denson, MD, Division of Gastroenterology Cincinnati Children’s, single cell multiome sequencing studies are carried out on intestinal biopsies from patients with AIH/PSC and inflammatory bowel disease.
Funding & Acknowledgements:
Cincinnati Children’s Research Foundation:
- ARC: Center for Translational Fibrosis Research
- ARC: Applying Deep Learning to Patient Classification of Inflammatory Liver and Gut Disorders
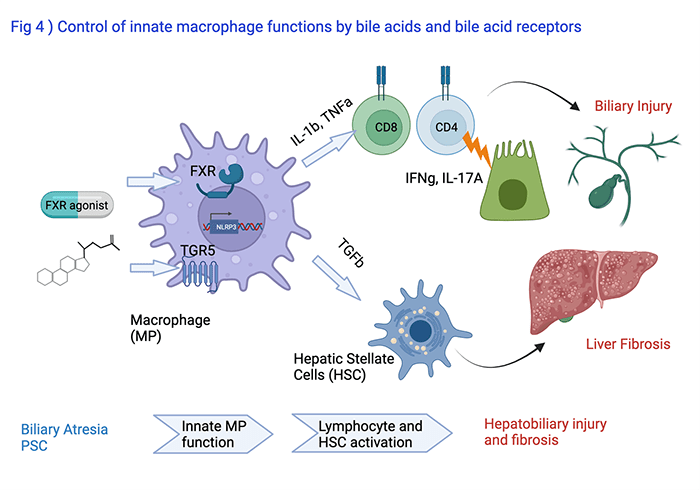
Modulation of Innate Macrophage Functions by Bile Acids and Bile Acid Receptors
Astha Malik, PhD, Research Associate, leads a program to uncover how the bile acid receptors FXR and TGR5 modulate the function of liver macrophages (Fig 4). These cells are important in orchestrating activation of lymphocytes and hepatic stellate cells which control bile duct epithelial injury and liver fibrosis, respectively, in conditions like biliary atresia or PSC. She presented her findings at the 2020 EASL and AASLD Annual Meetings.
Funding & Acknowledgements:
NIH/NIDDK
- R01 DK095001: Control of hepatic T cell responses in biliary atresia
- U01 DK62497: Clinical center for cholestatic liver disease in children
Cincinnati Children’s Research Foundation
- Center for Autoimmune Liver Disease
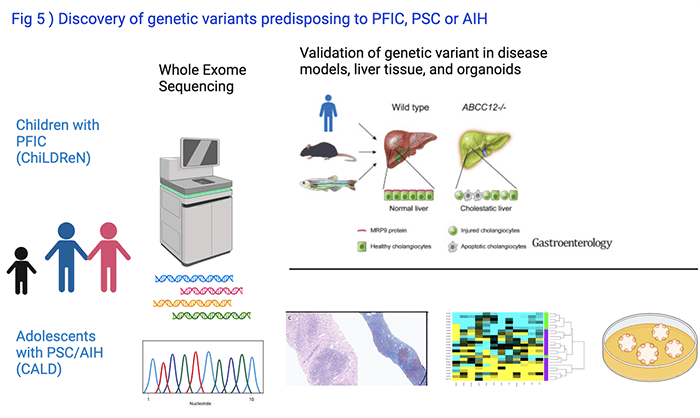
Genetic Studies in Children with Cholestasis
In collaboration with Alex Valencia, PhD, Lake Erie College of Osteopathic Medicine, and Chunyue Yin, PhD, Division of Gastroenterology at Cincinnati Children’s, we perform whole exome sequencing of DNA from patients with PFIC enrolled into the ChiLDreN network and from patients with PSC or AIH enrolled into CALD Biorepository (Fig 5). The relevance of novel genetic variants or of genes not previously linked to liver disease pathogenesis is explored in studies using model organisms like mice or zebrafish, circulating and liver derived immune cells, or pluripotent stem cell derived organoids. Our discovery of the role of the gene ABCC12 for maintenance of intrahepatic bile ducts was recently published in Gastroenterology.
Funding & Acknowledgements:
NIH/NIDDK
- R01 DK095001: Control of hepatic T cell responses in biliary atresia
- U01 DK62497: Clinical center for cholestatic liver disease in children
Cincinnati Children’s Research Foundation
- Center for Autoimmune Liver Disease
- Center for Pediatric Genomics
Investigating the Relationship Between Clinical Markers and Liver Disease
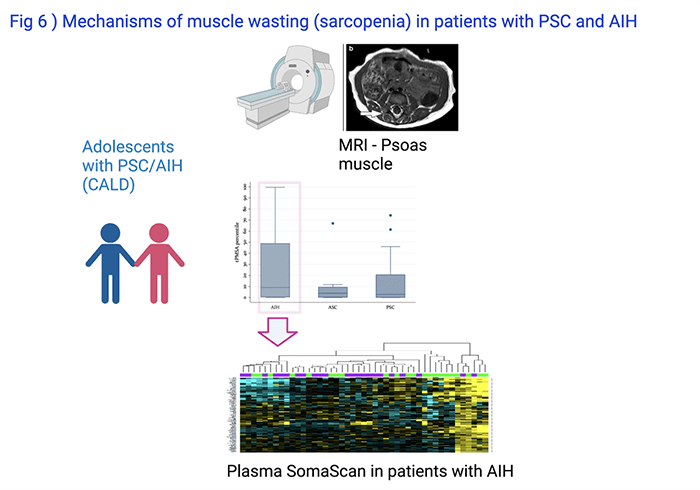
Muscle wasting (sarcopenia) is a well-known complication of aging, but also of chronic disease. In collaboration with Adjowa Amevor, MD, CNSC, and Marialena Mouzaki, MD, MSc, Division of Gastroenterology at Cincinnati Children’s, we found that sarcopenia, as assessed by measuring the psoas muscle surface area on research MRIs, affected almost 50% of patients with PSC or AIH enrolled into CALD Biorepository (Fig 6). It was associated with decreased health related quality of life (Amevor et al, Liver International, 2021). Ongoing studies on circulating plasma proteins and metabolites aim to understand the causes for sarcopenia in PSC and AIH.
Funding & Acknowledgements:
Cincinnati Children’s Research Foundation
- Center for Autoimmune Liver Disease
- Center for Translational Fibrosis Research
Novel Therapeutics
Pharmacological Inhibition of Intestinal Bile Acid Reuptake to Reduce Toxic Bile Injury in a Murine Model of PSC
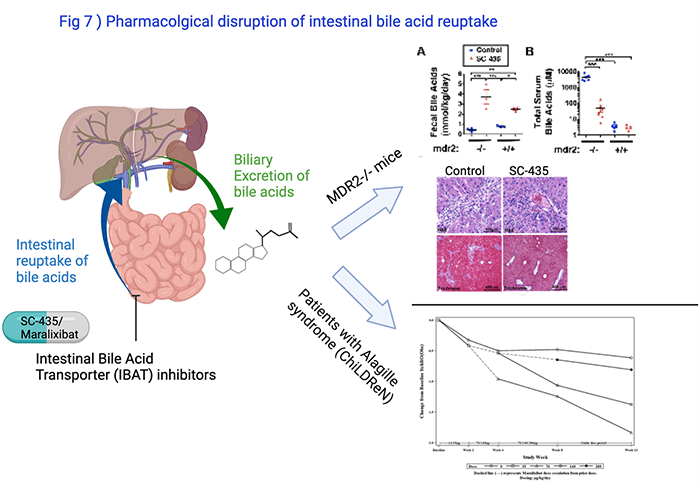
Bile acids, which are retained in the liver in diseases like biliary atresia or PSC, are key drivers of liver disease progression. Following excretion of bile acids from the liver they undergo reabsorption in the intestine. The transporter ASBT facilitates the reuptake in the terminal ileum. We showed that blocking ASBT with the small molecule SC-435 dramatically reduced serum and liver bile acid concentrations and improved the liver disease in MDR2-/- mice, a model of small duct PSC and biliary atresia after Kasai portoenterostomy (Fig 7). Ana Catalina Arce-Clachar, MD, found during her fellowship in Pediatric Gastroenterology that estrogen regulated ASBT expression in the intestine which worsened the liver disease in female MDR2-/- mice and rendered them more susceptible to the treatment with IBAT inhibitor compared with males. Studies sponsored by Mirum Inc. aim to find out whether combination of IBAT inhibitor with other anticholestatic therapies improves the response rate.
Studies in children with Alagille syndrome demonstrated that IBAT inhibitors reduced cholestatic itching in many of the patients (Shneider et al., Hepatol Commun, 2018).
Funding & Acknowledgements:
Mirum Inc:
- Randomized Double-blind Placebo-controlled Phase 3 Study to Evaluate the Efficacy and Safety of Maralixibat in the Treatment of Subjects with Progressive Familial Intrahepatic Cholestasis (PFIC) – MARCH-PFIC
- Assessing the efficacy of combination therapy with IBAT inhibitor in a preclinical model of sclerosing cholangitits
Prevention of Bile Obstruction in Mice
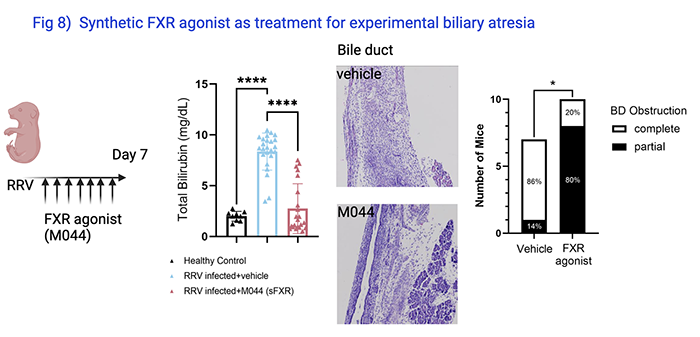
Annika Yang vom Hofe, Research Assistant, studies the efficacy of synthetic FXR agonist as treatment for biliary atresia. In an experimental model in which rhesus rotavirus induced bile duct obstruction in neonatal mice, administration of FXR agonist reduced serum total bilirubin level, a biomarker of biliary atresia in clinical trials, and prevented complete bile duct obstruction in most of the mice (Fig 8). The findings were presented at the 2021 AASLD Annual Meeting.
Funding & Acknowledgements:
In kind support by Metacrine
#BEAstrong fundraiser for research in biliary atresia
NIH/NIDDK:
- R01 DK095001: Control of hepatic T cell responses in biliary atresia
- U01 DK62497: Clinical center for cholestatic liver disease in children
Biomarkers
Assessment of Noninvasive MRI-based Biomarkers of Hepatobiliary Fibrosis in Autoimmune Liver Disease Patients
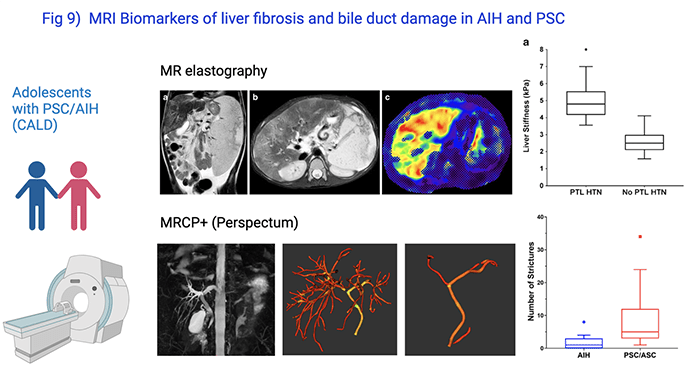
PSC and AIH are slowly progressive diseases. In pediatric PSC, most patients will improve initially after diagnosis, but over a period of 10 years, 50% will develop serious liver related complications. Biomarkers which can help to stratify patients according to risk for disease progression and which can serve as surrogate endpoints in short term clinical trials early in the disease process are urgently needed. Cyd Castro Rojas, PhD, and Mosab Alquraish, CLS/MBBS, lead our efforts of patient-based studies. They enroll patients with PSC or AIH in IRB-approved studies following informed consent, collect clinical data and biospecimen, and coordinate serial research MRIs. In collaboration with Jonathan Dillman, MD, MSc, and Andrew Trout, MD, Radiology at Cincinnati Children’s, and with investigators from Perspectum Diagnostics, we identified several MRI based non-invasive biomarkers to accurately predict biliary disease (sclerosing cholangitis) and liver fibrosis (Fig 9).
Funding & Acknowledgements:
NIH/NIDDK:
- U01 DK62497: Clinical center for cholestatic liver disease in children
Cincinnati Children’s Research Foundation
- Center for Autoimmune Liver Disease
- Center for Translational Fibrosis Research
Identifying Circulating Biomarkers Directly Related to Progression of Biliary Duct Injury and Fibrosis in Children
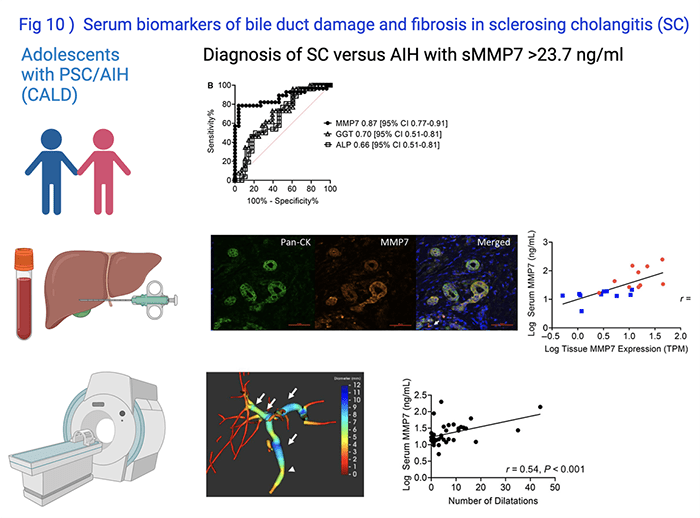
Simon Lam, MD, led investigations to determine the diagnostic accuracy of the serum MMP7 in predicting bile duct damage and biliary fibrosis and published his finding in Hepatology Communications in 2020 (Fig 10). This serum biomarker has the potential to be used as surrogate endpoint in clinical trials for pediatric PSC.
Funding & Acknowledgements:
Cincinnati Children’s Research Foundation
- Center for Autoimmune Liver Disease
- Center for Translational Fibrosis Research
- Cincinnati Digestive Health Center
Perspectum Diagnostics
Research Networks
Improving Care with an AILD-Specific Trailblazing Community of Patients, Parents and Experts
Biliary atresia, Alagille syndrome, PFIC, PSC, and AIH are rare diseases. Meaningful translational research and clinical trials to test the efficacy and safety of novel therapies require the infrastructure of multi-center research networks. Amy E. Taylor, MD, Division of Gastroenterology at Cincinnati Children’s, and Cyd Castro Rojas, PhD, in partnership with ImproveCareNow (ICN) lead the efforts to develop the Autoimmune Liver Network for Kids (A-LiNK), a Learning Health Network of 12 Children’s Hospitals across the US, to build an infrastructure for Quality Improvement and interventional trials to transform the care for children and young adults with PSC or AIH. Erin Chapman and Jennifer Hawkins, Clinical Research Coordinators, facilitate clinical studies on PSC in collaboration with ChiLDReN.
Funding & Acknowledgements:
Cincinnati Children’s Research Foundation
- Center for Autoimmune Liver Disease
NIH/NIDDK:
- U01 DK62497: Clinical center for cholestatic liver disease in children



