Investigating the Mechanisms of Airway Remodeling in Chronic Lung Disease
Airway wall thickening and epithelial injury are key features of small airway diseases, including chronic obstructive pulmonary disease (COPD) and bronchiolitis obliterans syndrome (BOS)—a late-onset, chronic complication following lung transplantation.
These structural changes contribute to airflow limitation and are major predictors of disease severity. Currently, effective treatments for COPD and BOS remain limited, underscoring the need to better understand the mechanisms driving epithelial loss and peribronchiolar fibrosis.
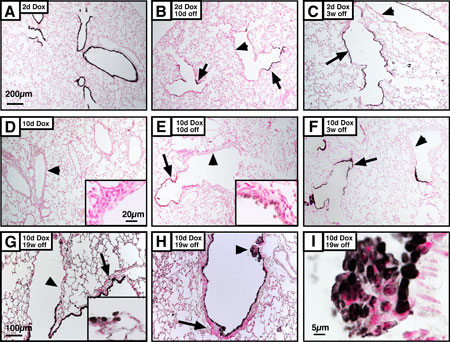
To investigate these processes, our laboratory developed a conditional transgenic mouse model that enables selective ablation of bronchiolar Clara (club) cells via diphtheria toxin expression. This model allows us to study both acute and chronic injury responses:
- After acute epithelial injury, the airway is temporarily covered by squamous epithelium, followed by regeneration of bronchiolar epithelium within approximately 10 days.
- Chronic injury, involving sustained Clara cell depletion and potential stem cell exhaustion, results in a persistent squamous epithelial phenotype and increased mesenchymal thickness, mimicking aspects of chronic airway disease.
This model provides a powerful platform for studying:
- The identity and role of bronchiolar stem/progenitor cells
- Cellular and molecular mechanisms driving epithelial repair following acute damage
- The pathogenesis of airway wall fibrosis in response to chronic epithelial injury.
Research Objectives
Our ongoing work aims to:- Define the signaling pathways and cellular programs that orchestrate bronchiolar regeneration.
- Identify mechanisms leading to aberrant repair and fibrosis, with the goal of informing novel therapeutic approaches for chronic airway diseases.