Lab Overview
The self-organizing tissue-based approach coupled with induced pluripotent stem cell (iPSC) technology has just begun as a promising field for designing a miniature organ, namely an organoid, in culture and is expected to achieve valuable outcomes in ‘(re-) generative medicine*’ and ‘drug development’1,2. However, how the complex but stereotyped tissue shapes self-organize still remains largely unknown. To understand such complex self-organizing mechanisms, conventional cell-level systems biology is simply not applicable as complete quantitative descriptions of the dynamic parameters of multicellular components and their interactions at biochemical, physical, genetic and epigenetic levels in space and time are completely unrealistic3.
Therefore, Dr Takebe’s lab proposes to take a ‘reverse reductionism approach’ for a holistic mechanistic understanding of the dynamic nature of a self-developing system. On the basis of cytosystems dynamics as elegantly summarized by Yoshiki Sasai3, we would like to achieve three interactive and complementary aims:
- The deductive development of a complex human organoid model
- The multidisciplinary dissection of self-driven mechanisms of organogenesis
- The technology prototyping towards drug discovery / transplant applications
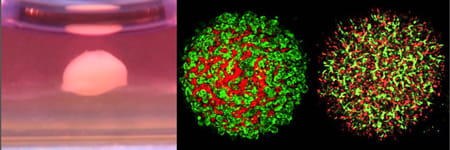
Our initial efforts were made on liver organoid (liver bud or miniature liver) systems using human iPSC4 (See Figure 1), followed by generation of other organoid systems from health and disease including cartilage, pancreas, kidney, lung, brain and cancer5. Towards a more complex and advanced organoid system generation, our lab studies direct or indirect contributions of previously untested supportive lineages and factors by deductive reconstitution of nature-mimicking environments in parallel mechanistic follow-up using mouse and human model. Additionally, by leveraging this new organoid model, we also seek to prototype a revolutionary technology platform for a practical use for human developmental biology, transplantation and drug discovery studies.
On-going examples of research topics are listed (but not limited to) below.
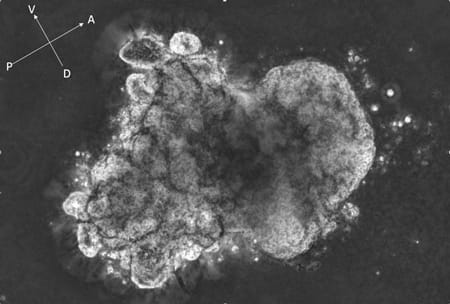
- Neighboring / distant organ support for endoderm organogenesis (See Figure 2)
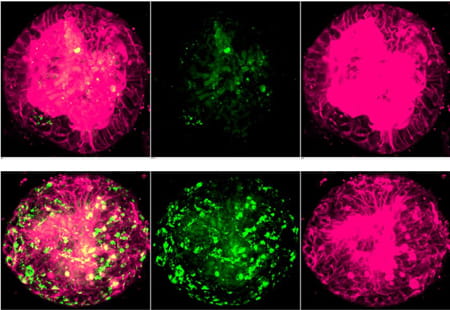
- Human fatty liver development model in an organoid format (See Figure 3)
- Inter-species difference on endoderm differentiation kinetics
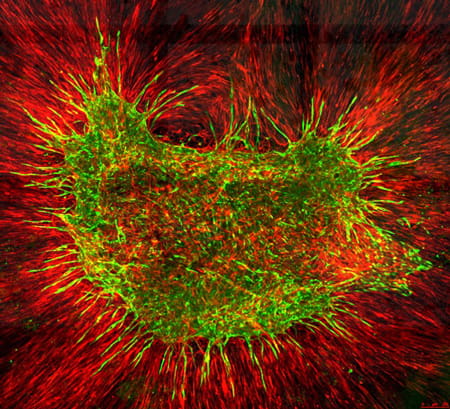
- Computational modelling of collective self-organizing behaviors (See Figure 4)
- Single cell based drug discovery technology
References
- Willyard C. The boom in mini stomachs, brains, breasts, kidneys and more. Nature. 2015 Jul 30;523(7562):520-2.
- Shinozawa T, Yoshikawa H-Y, Takebe T*; Reverse Engineering Organ Buds through Self-Driven Condensation and Organization. Developmental Biology 2016 Jun 27. pii: S0012-1606(16)30156-7. (*Correspondence)
- Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature. 2013 Jan17; 493 (7432):318-26.
- Takebe T*, Sekine K, Enomura M, Koike H, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H*: Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature, 499, 481–484, 2013. (*: Corresponding author)
- Takebe T*, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, Nakauchi H, Yoshikawa H-Y, Taniguchi H: Vascularized And Complex Organ Buds From Diverse Tissues Via Mesenchymal Cell-Driven Condensation. Cell Stem Cell, (*Corresponding author),16(5): 556-565, 2015