Current Projects
Current Projects
Antibodies such as IgG, IgE and IgA are important for normal host immune responses in serum and the mucosa. We are studying the molecular basis for antibody-mediated activation of immune receptors, inhibition of IgE-mediated anaphylaxis, and formation of immune complexes in autoimmune disease. These studies use a combination of biophysical and crystallographic techniques to study the structure of antibody:receptor complexes and to measure their affinity, stoichiometry, and kinetics of interaction. We are particularly interested in studying the formation of large pathological immune complexes by IgA1 and IgG in autoimmune diseases such as IgA nephropathy (Nature 2003; JASN 2011; Biochemistry 2008, 2010) and cryoglobulinemia (Nature 2015).
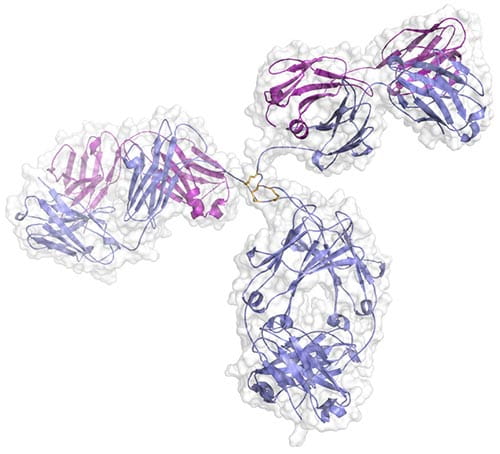
Crystal structure of a human IgG1 antibody. We are collaborating with Fred Finkelman and Rick Strait to study the differential role of antibody subtypes on autoimmune disease. Our groups recently showed that mouse IgG1 (analogous to human IgG4) plays a protective role in a mouse model of cryoglobulinemia (Strait, Nature 2015).
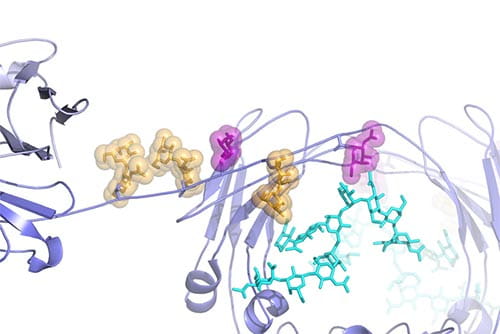
Structural model of the O-glycosylated hinge region of human IgA1 (Suzuki, JASN 2011; Herr, Nature 2003). Anti-glycan autoantibodies recognize undergalactosylated O-glycans in the IgA1 hinge to form immune complexes that become trapped in the mesangial region of the glomerulus, the filtration unit within the kidney. Deposition of these pathologic immune complexes eventually lead to end-stage renal disease in patients with IgA nephropathy.
Bacteria can grow in specialized, surface-adherent colonies known as biofilms. In this growth mode, the bacteria are highly resistant to antibiotics and immune responses. We are studying proteins responsible for holding the cells together in Staphylococcal biofilms. We have shown that a major biofilm protein from S. epidermidis called Aap self-assembles in the presence of zinc ions, forming ‘molecular velcro’ that mediates intercellular adhesion in the biofilm (PNAS 2008, 2013; Meth Enzymol 2011). We are studying this and other Staph proteins by X-ray crystallography and biophysical techniques to understand the molecular details underlying biofilm formation and to develop novel antimicrobial treatments. Currently, we are expanding into studying the microbiological aspects of mixed-species biofilm growth and comparing clinical Staph strains through next-generation genome sequencing.
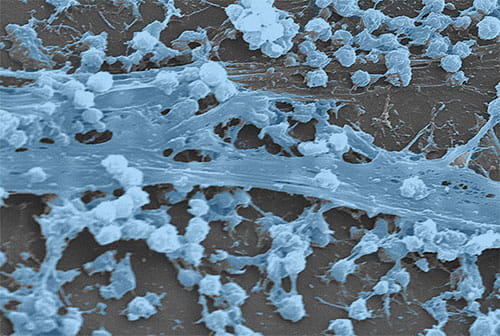
Staphylococcus aureus biofilm that has formed on an indwelling catheter (CDC Public Health Image Library)
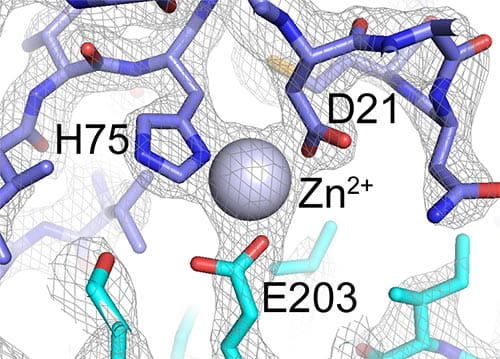
Zoomed-in view of the dimer interface between two B-repeat constructs from Aap. This view shows how the Zn2+ ion bridges two B-repeat proteins (shown in blue and cyan). The gray mesh shows the electron density map determined by X-ray crystallography.
In addition to studying the structure and function of the IgA-specific receptor FcαRI (CD89), our lab has a major focus on collagen-binding immune receptors. These are responsible for an interesting biological recognition event in which cell-surface receptors interact with a massive fibrous structure. We solved the structure of the platelet collagen receptor glycoprotein VI (GPVI) and are studying the nature of its interaction with collagen and other agonists (Blood 2006; Biochemistry 2009; JBC 2009). Given the important role of GPVI in thrombosis, we are pursuing structure-based inhibitors of platelet activation that target GPVI.
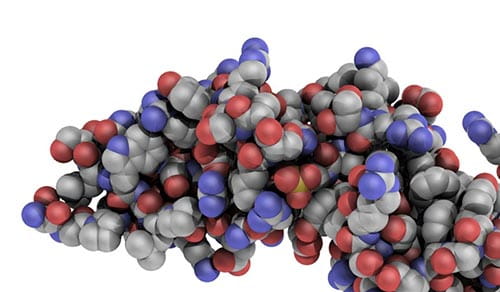
Atomic view of the N-terminal domain of GPVI.
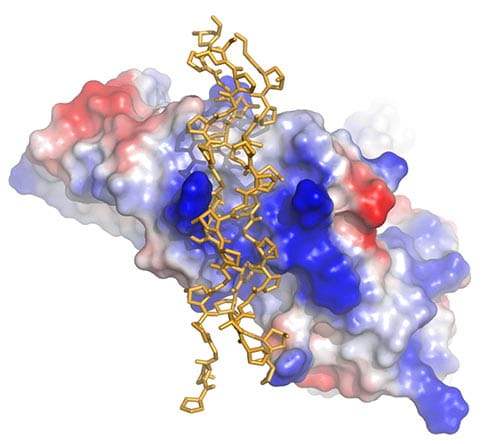
Bird’s-eye view of GPVI showing the groove that forms the binding site for the collagen triple helix (yellow sticks). The surface of GPVI is colored according to electrostatic potential.



