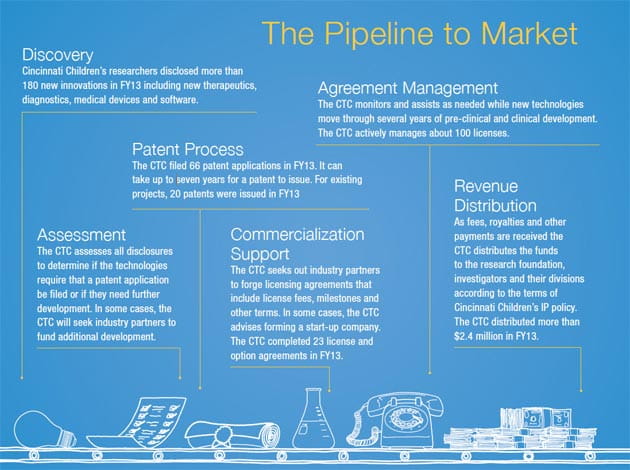
From Bright Idea to Brilliant Product
Our Center for Technology Commercialization helps scientists’ discoveries make the leap from laboratory to marketplace
Be it designing an infant-sized MRI scanner, developing a gene therapy for sickle cell disease or coming up with a better test for inflammatory bowel syndrome, clinicians and researchers at Cincinnati Children’s are constantly coming up with ways to improve pediatric medicine.
The challenge for our Center for Technology Commercialization (CTC) is to help translate a growing volume of innovative ideas into real-world products that benefit children – and to do it as quickly as possible.
“Our mission is clear: to be the leader in the commercialization of pediatric innovations,” says Niki Robinson, PhD, an Assistant Vice President at Cincinnati Children’s who leads the CTC.
Our need for expertise in technology transfer has grown in concert with the rapid expansion of our research enterprise. In the past 13 years, the medical center has pumped more than $1.5 billion in public and private grants into pursuing new discoveries. In fact, annual external research funding has more than tripled from about $50 million in 2000 to more than $171 million in FY2013, which ended June 30.
 |
|
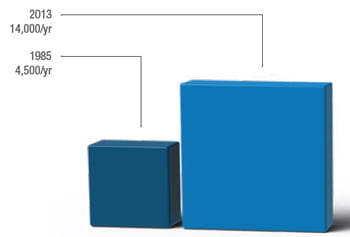
Scientists at Cincinnati Children’s have more than doubled their invention disclosures since 2009. |
Recent investments have spawned more than 200 new medicines, medical devices, diagnostic tests, software tools and other products now pushing their way through the development pipeline towards market. Hundreds more innovations are being disclosed every year.
The vital role the CTC plays in translating research into new therapies made the division the ideal candidate for becoming the first non-faculty division to participate in the medical center’s unique Scientific Advisory Committee (SAC) review process.
In the CTC review, experts from the Cleveland Clinic and Boston Children’s Hospital joined senior leaders at Cincinnati Children’s to analyze the division’s progress and strategic plans. The rigorous review produced two important outcomes:
 |
|
Cincinnati Children’s Innovation Fund invests nearly $1 million a year to support promising projects, says Dr. Niki Robinson, who leads the Center for Technology Commercialization. |
“The first thing we learned was that we are moving in the right direction at the right velocity,” Robinson says. “The second was that if we want to be the best at developing innovation, there is still more we can do.”
STAFF TO GROW WITH DEMAND
Seven years ago, the CTC had a professional staff of three. Now, its staff of 15 includes specialists in technology management, marketing and business development – and the CTC has six openings to fill. Plans include adding specialists with expertise in medical devices, healthcare information technology and other key fields.
EXPANDING EXTERNAL ENGAGEMENT
In addition to working directly with industry on a wide range of licensing and partnership arrangements, the CTC is expanding formal collaborations with other entities at regional and global levels.
A year ago, the division helped forge a new venture with Ben- Gurion University of the Negev in Israel to accelerate pediatric medical device development. Future collaborations may include leading a long-discussed statewide network for pediatric health innovation and joining an international rare diseases research consortium that was launched in 2011.
“Collaborating with industry and other research centers on product development is especially important in pediatrics because the research itself so often requires a non-traditional approach,” Robinson says.
ENHANCING INTERNAL ENGAGEMENT
Cincinnati Children’s made a strong statement last year by launching an Innovation Fund to accelerate marketable projects. The fund plans to invest $500,000 to $1 million a year in promising projects that need proof-of-concept funding to attract commercial partners.
Very few pediatric centers are large enough to make such investments, but such funding is becoming more important, Robinson says. Large pharmaceutical companies and device makers have become less willing to take financial risks to develop products for rare childhood disorders with inherently small market potential.
Now that the Innovation Fund has been established, it is encouraging an entrepreneurial spirit among our faculty, Robinson says. The proof? The number of proposals was twice as high for our second round of Innovation Fund awards compared to the first round.
NNOVATION FUND PICKS 6
These researchers were selected from more than 30 applications in FY13 to receiving support to prepare their recent discoveries for commercialization.
Lee (Ted) Denson, MD, Gastroenterology – To develop a novel diagnostic tool that uses genetic signatures and computer algorithms to more accurately determine whether patients have Crohn’s disease or ulcerative colitis.
Charles (Chuck) Dumoulin, PhD, Radiology– To continue developing a novel small-scale MRI scanner that will provide quieter, improved images of newborns – especially fragile premature infants – without removing them from the neonatal ICU.
William (Bill) Hardie, MD, Pulmonary Medicine – To further develop an oral therapy that would reverse pulmonary fibrosis – a potentially fatal complication of several adult and pediatric diseases -- by targeting a specific molecule (P70S6K).
Punam Malik, MD, Experimental Hematology/ Cancer Biology – To continue study of an improved y-globin gene developed by Malik’s research team for a Phase I/II clinical trial of gene therapy for sickle cell disease.
Senthil Sadhasivam, MD, MPH, Anesthesia – To develop a rapid genetic test and decision support tool that would allow physicians to precisely tailor the use of opioids for pain management.
Hector Wong, MD, Critical Care Medicine – To continue validating a novel diagnostic biomarker (IL-27) for rapid, early identification of sepsis, which could prevent potentially fatal septic shock by allowing earlier intervention with antibiotics.