Gene Study Reveals Which Patients with Shwachman-Diamond Syndrome are at High Risk for Leukemia
Published February 2021 | Nature Communications
A recent translational study provides insight into the genetic underpinnings of leukemic development in Shwachman-Diamond Syndrome (SDS). Investigators anticipate that their findings could lead to better screening strategies, new diagnostic tests and targeted therapies.
SDS is a rare, inherited disease predominantly caused by biallelic germline mutations in the SBDS gene. In SDS, ribosomes do not assemble correctly, leading to co-morbidities including bone marrow failure, pancreatic insufficiency, and other issues. About 40% of people with SDS develop myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), usually in early adulthood.
“Survival of people with SDS who develop blood cancers is poor because often if the disease is that advanced, even a bone marrow transplant may not be curative,” says Kasiani Myers, MD, a pediatric hematologist and researcher with the Division of Bone Marrow Transplantation and Immune Deficiency at Cincinnati Children’s. “If we knew a child with SDS had developed higher-risk features concerning for MDS or AML development, we could do an early allogeneic stem cell transplant and potentially save their life.”
Samples from 110 SDS patients tested in the study came from the North American Shwachman-Diamond Syndrome Registry, which Myers co-directs with Akiko Shimamura, MD, PhD, at Boston Children’s.
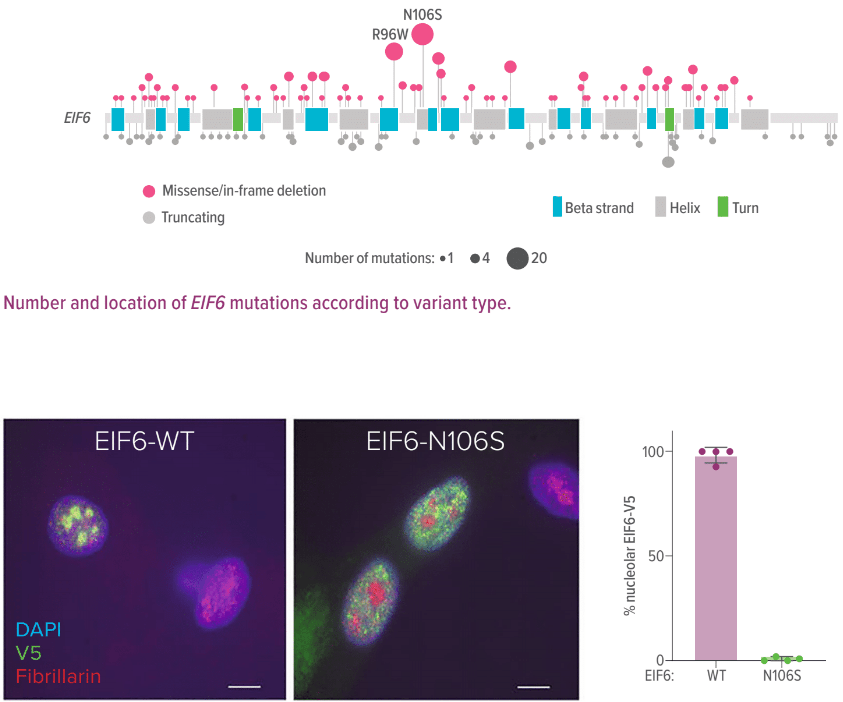
Researchers observed longitudinal changes in somatic mutations that lead to leukemic development—in some cases, changes over 10 years. The most commonly and independently mutated genes were EIF6 and TP53. The team also found that the presence of TP53 mutations in both alleles is strongly associated with leukemic development.
Clinical laboratories do not test for the biallelic TP53 gene mutation. Myers and colleagues hope to develop a clinical test based on next-generation sequencing that would allow physicians to track changes in the mutated genes.
Immunofluorescence of V5-EIF-WT or V5-N106S-EIF6 protein in SDS patient-derived fibroblasts, V5 (green), fibrillarin (red), and DAPI (blue). Right panel: quantification of V5 nucleolar signal from four independent experiments. Error bars represent the mean ± standard deviation. Scale bar = 10 μm