The study of the link between abnormalities in redox dysregulation and inflammation in CF
Inflammation in the cystic fibrosis (CF) lung has been shown to be excessive, and slow to resolve. Typically, this leads to lung damage, eventual lung failure and is the leading cause of mortality in CF. A number of studies have reported that CF cells, especially airway epithelia produce abnormal levels of inflammatory cytokines in response to inflammatory stimuli. Anti-inflammatory therapy that controls inflammatory signaling has been shown to be beneficial, slowing lung deterioration in patients, and is perhaps one of the best therapies for CF.
Therefore, delineating the mechanisms of inflammation in CF, which can yield targets for anti-inflammatory therapy, is an important and worthwhile effort. Furthermore, it is logical to suspect that downstream mechanisms contributing to inflammation in the CF lung are also present in other inflammatory lung diseases, such COPD and Asthma, increasing the significance of studying aberrant inflammatory signaling in CF. However, neither the mechanisms that promote nor those that limit inflammatory responses are well understood.
Our lab is the first to discover that an intracellular redox imbalance in CF epithelia was caused in part by the dysregulation of a very important transcription factor, nuclear-factor-E2-related factor 2 (Nrf2), the central transcription factor in the antioxidant response element in cells (Figure 1).
Recent studies by our lab have revealed a dysregulation of the antioxidant response element (ARE) transcription factor, Nrf2, in multiple in vitro and in vivo models of CF (Figure 1). Although CF cells exhibit oxidative stress, Nrf2 protective cascades are not activated and are in fact decreased (Figure 2). This results in the accumulation of intracellular oxidants, which significantly increases inflammatory cytokine production and reduces the activity of pathways that resolve inflammation. This is an important finding as Nrf2 has been implicated in a number of inflammatory lung diseases and can be safely and specifically activated, and, is therefore, a viable therapeutic target (Figure 3). Rescue of Nrf2 dysfunction in CF epithelial cells reduces inflammatory responses to normal levels, but does not inhibit normal responses, which would be deleterious in the context of CF.
 |
|
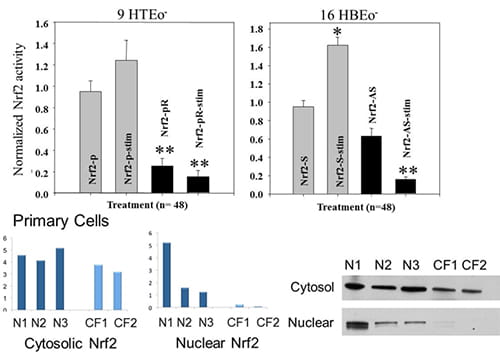
| Figure 1. A. Nrf2 expression and activity are decreased in CF epithelia in the absence and presence of inflammatory stimulation. Normal and CF matched cell pairs are co-transfected with a firefly luc plasmid (Nrf-2 promoter) and a control Renilla luc plasmid (CMV promoter). *connotes significant difference from unstimulated normal control (p < 0.05), while **connotes significant difference from both unstimulated and stimulated normal control. Each data bar represents the average of 8 replicate wells in 4 experiments. (doi:10.1371/journal.pone.0003367.g002)B. Rp-cAMPS modulates nuclear Nrf2 levels in CF airway epithelia. The impact of Rp-cAMPs on Nrf2 was examined. Cells were maintained in the absence or presence of 50 µM Rp-cAMPs for 72 h. Cytoplasmic and nuclear fractions of cells were homogenized, subjected to SDS-PAGE, transferred to PVDF membrane, and probed for Nrf2 by Western blot. Gel wells are loaded with equal amounts of protein (20 µg for cytoplasmic fractions and 10 µg for nuclear fractions). Band intensities were normalized to β-actin levels and then to respective untreated non-CF controls (control value set as 1). Nrf2 levels in human primary airway epithelia (n = 3 different donors under each condition). All samples were analyzed at least 3 times on different gels. Representative gels shown. *Significant difference (p < 0.05) from non-CF control. **Significant difference (p < 0.05) from untreated CF cells. Error bars represent SE. (doi: 10.1152/ajplung.00156.2011) |
|
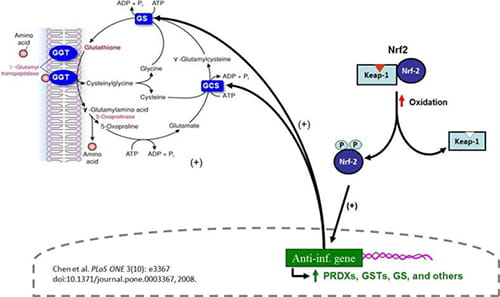 |
Figure 2. Nrf2 boosts anti-inflammatory signaling, in part by controlling oxidant levels in cells. |
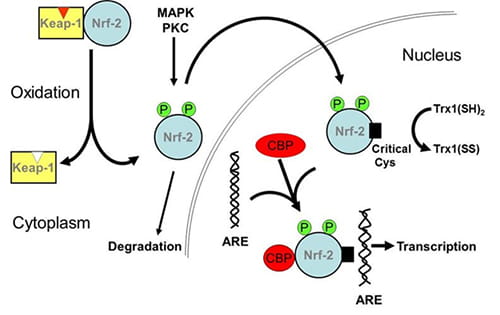
Figure 3. Schematic of the Nrf2 activation of cascade. |
 |
Our chief interest is in the question of how CFTR dysfunction gives rise to aberrant inflammatory signaling in the airways. Our lab was the first to describe a dysfunction in Nrf2 in CF epithelia, and already we have shown that modulation of Nrf2 activity markedly impacts inflammatory signaling in CF. More recently the lab has described at least part of the mechanism of downregulation of Nrf2, which stems from feedback responses to the loss of CFTR function. These studies on mechanism are the first to link a secondary defect in CF cells to CFTR dysfunction.
A postdoc in our lab is examining the regulation of Nrf2 activity in CF primary epithelial cells, CF animal models, and tissues from CF patients.
We plan to:
- Determine the step or steps in the Nrf2 activation cascade that are dysfunctional in CF;
- Examine the mechanism by which CFTR dysfunction results in the dysregulation of Nrf2;
- Test pharmacological agents which activate Nrf2 by different mechanisms to elucidate potential therapies for Nrf2 dysfunction.
These studies have the potential to delineate the link between CFTR dysfunction and inflammation in CF, and define novel therapeutic targets. Furthermore, our studies extend to mechanisms of inflammation and disease observed in cardiac (foam cell formation and arthrosclerosis), pulmonary (chronic obstructive pulmonary disease and asthma), and neurological (Niemann-Pick and Parkinson’s disease) disorders.



