Web-Based Tool Helps Manage Methotrexate Toxicity Risk
Published September 2020 | Clinical Pharmacology & Therapeutics
Some children and adults with acute lymphoblastic leukemia (ALL) face a risk of toxicity from high-dose treatment with methotrexate (MTX) because their bodies are slower than others at clearing the drug from their systems.
Severely delayed MTX clearance is seen in up to 1% of pediatric patients with ALL. If this metabolic difference is not detected and managed, patients can experience life-threatening kidney, liver and intestinal damage in addition to their cancer. While folinic acid often helps the kidneys to eliminate MTX, sometimes patients need a more powerful drug, glucarpidase. This rescue agent can eliminate 90% of MTX concentrations within 15 minutes, so it should be administered only when MTX concentrations exceed specific parameters. Otherwise, there’s a risk of cancer relapse because too much MTX can be neutralized.
“There has been a lack of clinical tools to identify patients likely to experience delayed MTX clearance. As a result, response tends to be reactive,” says corresponding author Laura Ramsey, PhD. “Now we have a tool that can be used early in a course of treatment.”
To address this concern, a team of researchers with the Division of Research in Patient Services used data from 772 children receiving MTX to develop a new pharmacokinetic (PK) model that better defines the clearance curves for patients based on several clinical factors. That model now supports a web-based clinical decision support tool called MTXPK.org.
“This tool can help clinicians optimize the administration of glucarpidase, which is indicated only when both the MTX concentration and an increase in creatinine levels reach specific targets,” says first author Zachary Taylor, then a graduate student in the Ramsey Lab.
The new tool blends individualized demographics, with serum creatinine measures, and real-time drug concentrations to predict a patient’s MTX elimination profile.
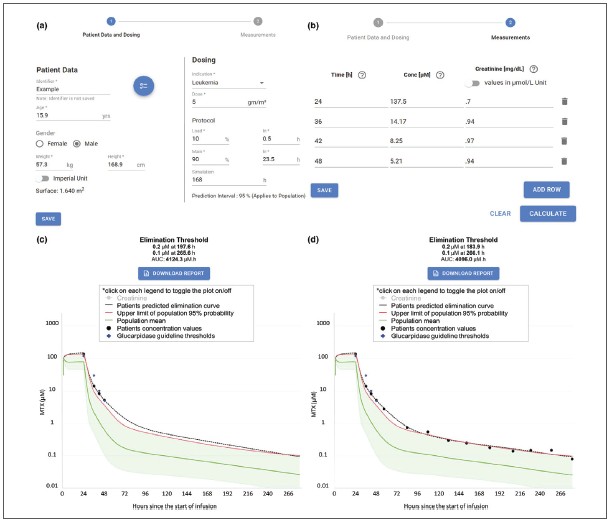
How the MTXPK.org Decision Support Tool Works
Once patient demographics, plasma methotrexate (MTX) concentrations and serum creatinine levels are loaded, the tool calculates an individualized pharmacokinetic (PK) concentration-time curve. In this demonstration case, the first screen (c) shows a patient that meets indications for glucarpidase. The second screen (d) shows the same patient after administering glucarpidase, demonstrating the accuracy of the predicted curve. Black line shows the individual elimination curve; green line shows population elimination curve; red line shows 2 standard deviations above the population curve and the purple diamonds show glucarpidase guideline thresholds.