Current Projects
Current Projects
We have two major projects investigating the role of Bcl2 family members in T cell homeostasis. One project focuses on T cell development in the thymus. Central (thymic) tolerance hones the T cell repertoire to be both self-tolerant and MHC-restricted through processes of positive and negative selection. However, some thymocytes are thought to avoid negative selection as they react with appropriate affinity / avidity to their peptide ligands. Such selected cells include Natural killer T (NKT) cells, FoxP3+ regulatory T cells and the precursors of TCRβ+ CD8αα+ intraepithelial lymphocytes (IELs) and are collectively referred to as “agonist” selection cells. Our prior work showed that the pro-apoptotic protein, Bim controls the development of agonist selected cells (Li et al J. Immunol. 2017). Here, we investigated the role of anti-apoptotic Bcl-2 family members, Bcl-2, Bcl-2A1, and BclxL in promoting the central development and peripheral survival of agonist selected cells.
A second project investigates the development of T cell memory. Maintenance of T cell homeostasis is critical for a normal functioning immune system. T cell Transient disruption of homeostasis occurs when naïve T cells undergo antigen-driven expansion and acquire effector functions. Effector T cells then either undergo apoptosis or survive to become memory cells. This life / death decision is crucial: it resets T cell homeostasis, promotes protective immunity, and limits autoimmunity. However, the mechanism(s) underlying this cell-fate decision remain unclear. The critical downstream executioner of this effector T cell death program is the pro-apoptotic Bcl-2 family member, Bim. Mice lacking Bim have a sustained and profound survival of antigen-specific T cells following antigenic challenge. Although the current model suggests that interference with death should not affect memory generation (effector cells should not acquire the pre-memory genetic program) our investigation of Bim function suggests otherwise.
We are currently working to determine the degree to which cells that have avoided Bim-driven death can acquire a memory phenotype.
Funding: R01 AI057753 - Regulation of Apoptosis in Activated Primary T Cells. June 1st, 2005 – Nov 30th, 2015. NIH/NIAID. PI: Hildeman.
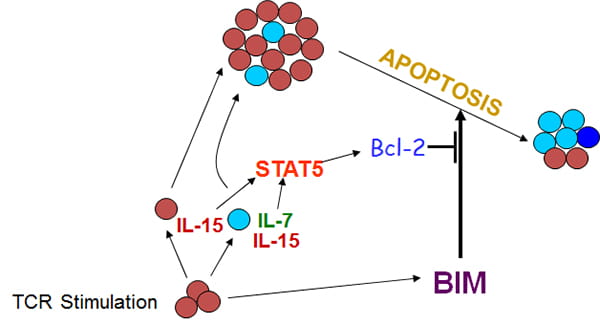
A cytokine-Stat5-Bcl-2 pathway antagonizes Bim and controls effector CD8+ T cell survival.
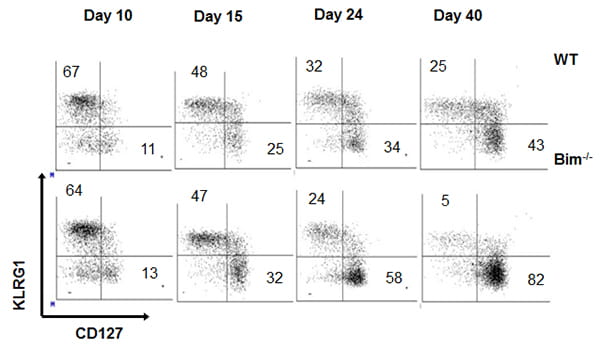
Do Bim-deficient T cells acquire the genetic program that governs memory T cell development?
Age-related inflammation (so-called inflammaging) is associated with many deleterious processes in the elderly, including Alzheimer’s disease, cardiovascular disease, and general frailty. Declining immune function is also well described in the elderly, and leads to increased risk and severity of infection, poorer control of cancer, and impaired responses to vaccination. While there is clearly cell-intrinsic immune dysfunction with age, our and others data have revealed that cell extrinsic mechanisms also play significant roles.
Our long-term goal is to understand the mechanisms underlying the dysregulation of immune responses in aging and develop therapeutic strategies to enhance protective immune responses in the elderly.
In close collaboration with the Chougnet lab, we have discovered that two distinct types of regulatory T cells accumulate with age, classical FoxP3+ Tregs and a novel population of IL-10-secreting CD4+ T follicular helper cells. Current studies are investigating the accumulation and function of both of these cells in aged mice and humans.
In addition to accumulation of suppressive T cell populations, aging is also associated with decreased function of antigen-presenting cells, including dendritic cells (DCs). A key aspect of DC function is their capacity to “cross-present” cell-associated Ag to CD8+T cells, a process that is required to cross-prime CD8+T cell responses against tumors or intra-cellular pathogens. However, the effect of aging on the acquisition, processing, and presentation of cell-associated Ags by primary DCs has barely been studied. We recently uncovered that aging significantly reduced the cross-presenting and cross-priming capacity of the DC subsets the most efficient at carrying out this important task (CD8αDCs and merocytic DCs). Current studies will investigate mechanisms that control defective antigen uptake and processing in aged DCs.
Funding:
RO1 AG033057 - Homeostasis and function of regulatory T cells in aging. Sept 15, 2009 - July 31st, 2017. NIH/NIA. Co-PIs: Chougnet, Hildeman
RO1 AG053498 - Metabolic alterations in age-associated dendritic cell dysfunction. Co-PIs: Chougnet, Hildeman
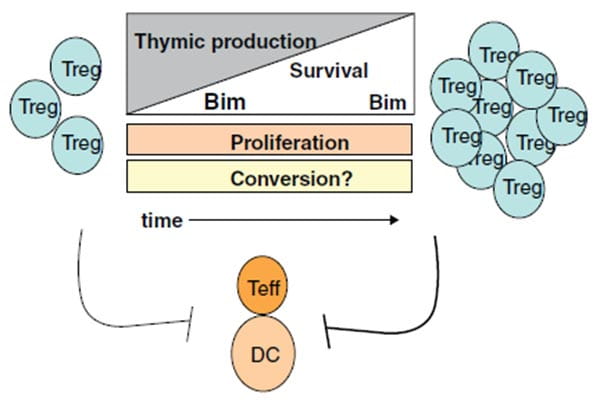
Mechanisms underlying Treg accumulation with age.
The ultimate goal of organ transplantation is to achieve sustained allograft acceptance with minimal broad-based immunosuppression and off-target toxicity. Current therapies in renal transplantation involve an induction step (non-specific T cell depletion) followed by long-term maintenance immunosuppression with calcineurin inhibitors (CNI) and corticosteroids (CCS). Despite the effectiveness of these therapies, their long-term usage leads to nephrotoxicity, cardiovascular and metabolic effects that can directly contribute to allograft loss. Thus, there is an urgent need to maintain long-term allograft tolerance, without substantial nephrotoxicity. We have two major projections in close collaboration with transplant surgeon Dr. E Steve Woodle, the lab has two major projections:
- analysis of T cell mediated rejection via single cell analysis of biopsies
- understanding allo-specific plasma cells that drive donor-specific antibodies that drive chronic rejection
Both projects involve acquisition of tissue biopsies from patients treated with novel therapeutics in clinical trials.
Funding: R21AI142264 Single cell analysis of transplant rejection, NIH, NIAID, PI: Hildeman. Grants from Sanofi and Novartis



