Vasculature and Stem Cell Niches
Blood vessels, which are lined by specific cells called endothelial cells, are typically known for their roles in transporting oxygen and metabolites to organs throughout the body. However, in the past 20 years, a greater appreciation has arisen for vasculature’s instructive and developmental roles during organogenesis and organ homeostasis. For example, studies in mice have demonstrated that juxtacrine and paracrine signals from endothelial cells are critical for establishment and maintenance of stem cell niches, such as in neural and hematopoietic tissues.
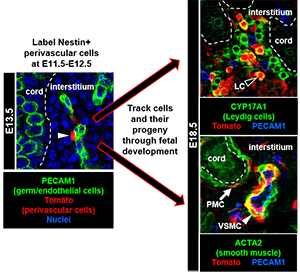
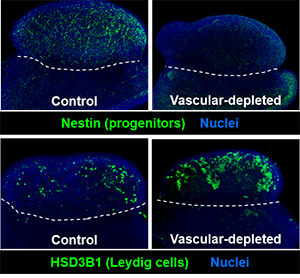
Previous research by our group and other groups has shown that vasculature is one of the earliest hallmarks of sex-specific gonadal differentiation, in which a new arterial network is generated only in fetal testes. Our work has revealed that blood vessels in the fetal testis are a vital component of the niche for perivascular stem/progenitor cells that give rise to steroid-producing Leydig cells, smooth muscle cells, pericytes, and potentially other fibroblast-like cell types. Our research now has expanded to addressing the role of vasculature and perivascular cells in the ovary, which is a relatively under-appreciated aspect of ovarian physiology. We are also currently studying the mechanisms that drive sex-specific vascular formation, as well as how blood vessels interact with mesenchymal perivascular cells, the latter of which are poorly understood but have been implicated as a multipotent cell type involved in developmental and pathological processes.