Registry Helps Team Discover 7th Gene Linked to Rare Blood Disorder
Published November 2020 | American Journal of Human Genetics
Congenital dyserythropoietic anemia (CDA) is so rare that specialists in blood disorders rarely encounter a patient. But thanks to a recently launched registry covering all of North America, scientists have gathered enough data to identify a seventh gene associated with the disease.
People with CDA cannot produce red blood cells normally, which results in anemia, excessive iron levels, and organ damage. Previous studies have identified six gene variants associated with the condition. But these explain about half of known cases.
By exploring the Congenital Dyserythropoietic Anemia (CDA) Registry—launched in 2016 with funding from the Cincinnati Children’s Center for Pediatric Genomics—a team led by first author Katie Seu, PhD, and senior author Theodosia Kalfa, MD, PhD, found three unrelated people with mutations of the gene VPS4A, but none of the other six CDA-associated genes.
This gene helps regulate cellular processes, including cell division and endosomal vesicle trafficking. In CDA, the variant resulted in red blood cell precursors with distinctive double nuclei and cytoplasmic bridges that bound cells together even after they divided.
Two of the three affected children with VPS4A variants in the ATPase domain were born with microcephaly and significant brain development dysfunctions. The third child carrying VPS4A variants in another domain showed milder symptoms. Analyzing the genomic data required numerous steps, ultimately including whole-exome sequencing and deriving induced pluripotent stem cells (iPSCs) to model the syndrome. With this process established, researchers hope to find more variants linked to CDA or other rare blood disorders, the co-authors say.
“Our findings demonstrate that normal function of VPS4A is essential for human erythropoiesis,” Kalfa says. “VPS4A mutations cause cytokinesis and trafficking defects leading to a human disease with detrimental effects to neurodevelopment and red cell production.”
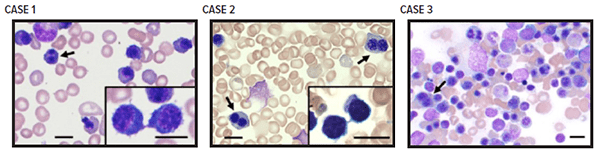
These bone marrow aspirate smears, collected from a patient registry launched in 2016, show erythroid hyperplasia and dysplasia associated with a variant of the gene VPS4A. Erythroblasts (blue stain) show megaloblastoid changes and include cells with binucleation (arrows) and cytoplasmic bridges joining erythroblasts post-division, noted especially in probands 1 and 2 (insets).





