First Human Esophagus Organoid Grown from Pluripotent Stem Cells
|
Top Breakthrough Discovery | Published September 2018 in Cell Stem Cell |

Stephen Trisno
The first human esophageal tissues grown entirely from pluripotent stem cells (PSCs) add to a significant track record of breakthroughs in organoid development at Cincinnati Children’s and our Center for Stem Cell and Organoid Medicine (CuSTOM).
The project was led by James Wells, PhD, senior author, and first author Stephen Trisno, a graduate student in Wells’ lab. Other Cincinnati Children’s scientists on the project included Katherine Philo, Emily Catá, Sonya Ruiz-Torres, Scott Rankin, Lu Han, Talia Nasr, Praneet Chaturvedi, Marc Rothenberg, MD, PhD, Susanne Wells, PhD, and Aaron Zorn, PhD.
“In addition to being a new model to study birth defects like esophageal atresia, the organoids can be used to study diseases like eosinophilic esophagitis (EoE) and Barrett’s metaplasia, or to bioengineer genetically matched esophageal tissue for individual patients,” Wells says.
A step forward for rare conditions
Functional organoid tissue can allow investigators to learn much more about the genetic and biochemical mechanisms underlying many esophageal conditions, including congenital defects like esophageal atresia and later-life conditions such as esophageal cancer, gastroesophageal reflux disease (GERD), or achalasia, which prevents the esophageal contractions needed to pass food.
“In moving forward we are using similar approaches to build additional tissue complexity and functionality.”
Potential applications of this new technology range from using organoid tissues to test drug toxicity in a human system, to developing custom-edited versions that mimic specific conditions, to eventually growing enough tissue for transplantation.
In this study, the team grew esophageal organoids to lengths of 300-800 micrometers, which required about two months each. Comparisons to patient biopsies showed that the bioengineered tissues were strikingly similar at the biochemical level. However, these organoids are not yet large enough for tissue transplantation.
“These tissues would have to be a couple orders of magnitude larger (on the centimeter level) to likely be useful in transplantation,” Trisno says. “To get to that level, it will likely require incorporating other cell types that can form blood vessels, muscles, and nerves to support larger tissues sizes.”
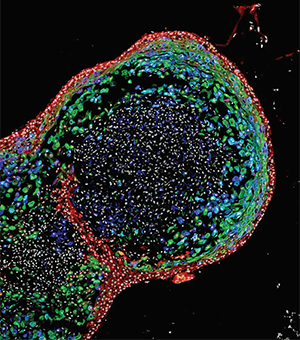
This confocal image of an esophageal organoid was featured in National Geographic in September 2018.
Vital role for Sox2
Starting with human PSCs, the team guided the cells through a series of precisely timed manipulations of genetic and biochemical signals that pushed them to form embryonic endoderm and foregut tissues. Then they focused in on the gene Sox2.
Based on previous work involving frogs, mice and human tissue cultures, the team determined that the Sox2 protein inhibits Wnt signaling at a critical point in early development, which in turn prompts the cells to form esophageal tissues rather than other airway tissues. To confirm the gene’s role, the team developed a Sox2 knockout mouse, which resulted in mice born with esophageal agenesis—in which the esophagus terminates in a pouch.
“This finding was not a complete surprise, as other studies had shown that deficiencies in Sox2 caused defects in esophagus development,” Wells says. “However, the mouse model we used was cleaner and more thorough in wiping out Sox2 from the developing endoderm, which is what yielded a different result. Additionally, we were able to complement the mouse experiments using the human PSCs to begin to understand how Sox2 is controlling esophageal development.
Another success
Cincinnati Children’s has had several successes in organoid development, dating back to a paper in Nature Medicine in 2010 reporting the formation of functional intestinal tissue. Other advances since then include creating organoids for the stomach, colon, and liver; and demonstrating a method for adding nerve function to intestine organoids.
In November 2017, the medical center launched CuSTOM to build on these discoveries and help move them toward the clinic.
“The next steps for these organoids would be to apply this model to study other aspects of esophageal development and diseases,” Wells says. “In moving forward we are using similar approaches to build additional tissue complexity and functionality and to scale up the size of these tissue constructs so that we can test them as therapeutic transplants in animal models of esophageal disease.”
Citation
Trisno SL, Philo KED, McCracken KW, Cata EM, Ruiz-Torres S, Rankin SA, Han L, Nasr T, Chaturvedi P, Rothenberg ME, Mandegar MA, Wells SI, Zorn AM, Wells JM. Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell Stem Cell. 2018 Oct 4;23(4):501-515.e7.



