Targeting RipIL-33 Pathway Could Transform Allergy Treatment
Published October 2021 | Nature Immunology
Despite many years of research and the development of a number of medications intended to help control common allergies, only some people find consistent relief from the sudden inflammation of airways, the itchy eyes and other symptoms when they encounter the allergens plaguing them.
Millions of people have allergies because so many aspects of our environment can trigger reactions, be it ragweed, mold, pet dander, insects, tree pollen and more. Yet medications that help one form of allergy often do not help against another, in large part because the precise molecular mechanisms that drive immune response to allergens have remained elusive.
Now, the discovery of a built-in rapid reaction system that triggers inflammatory responses when people are exposed to a large set of common allergens may open the door to dramatically improved treatments. The findings focus on the responses to allergens from insects, mites and fungi, which turn out to differ significantly from allergic responses to plant pollens or food.
The study, led by Cincinnati Children’s scientists Michael Brusilovsky, MMedSc, PhD, Chandrashekhar Pasare, DVM, PhD, and Marc Rothenberg, MD, PhD, reveals unexpected new details about how the type 2 innate immune response system works.
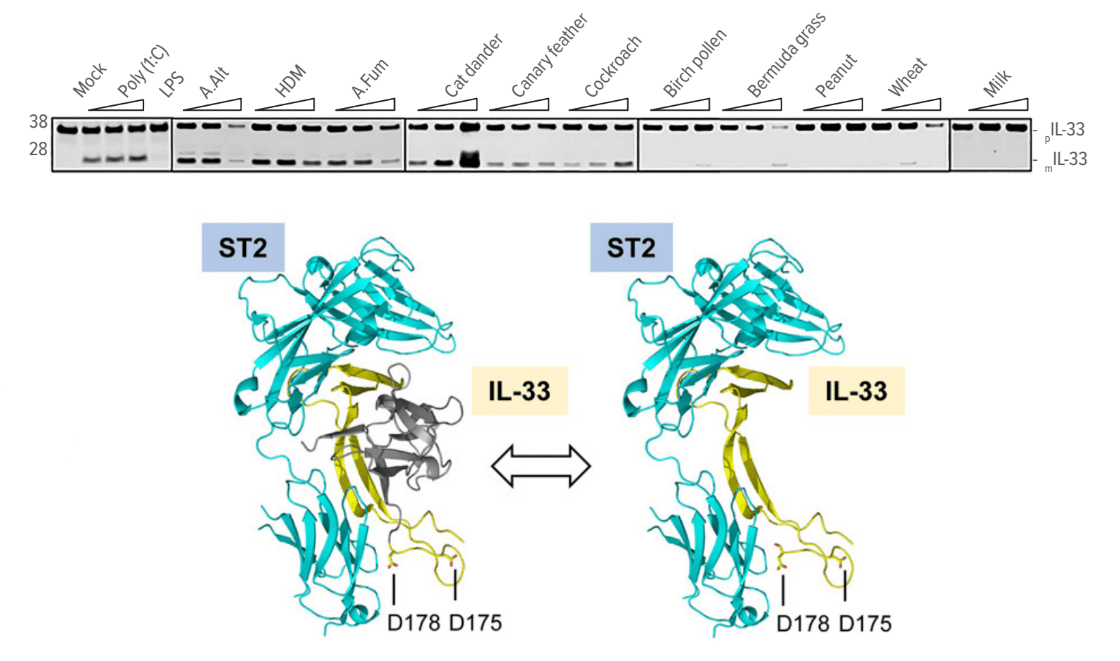
Among the many experiments involved, the team compared responses at a molecular level to several types of allergy-inducing exposures. This work included exposing mice to substances including house dust mite, A. fumigatus, cat dander, canary feathers, cockroach, birch pollen, Bermuda grass, peanut, whole wheat, and cow milk extracts.
Ultimately, the team determined that a common biological response platform kicks in to rapidly release inflammation-causing IL-13 in response to allergens from cockroaches, mites and fungi—but not to food allergens or plant pollens.
“Disrupting this allergen-sensing pathway could provide a unique opportunity to counteract type 2 immunity and alleviate allergic inflammation,” Rothenberg says.
In theory, any new medication that proves capable of controlling this response pathway could benefit people suffering from a wide range of allergies.
Tracking the RipIL-33 Pathway
Top: How common allergens activate the RipIL-33 response pathway.
Bottom: Ribbon diagram demonstrates that IL-33 processed via the RipIL-33 pathway is biologically active and binds its cognate receptor ST2.
Far-Reaching Implications
The findings impressed other experts in immune response, who co-authored a commentary in Cellular & Molecular Immunology about the work.
“The authors elegantly describe how epithelial cells contribute to the onset of allergic airway inflammation by activating the ripoptosome, which subsequently drives the maturation and secretion of IL-33,” states the editorial written by Jutta Horejs-Höck, PhD, University of Salzburg, and colleagues Theresa Neuper and Richard Weiss.
“First, the authors showed that allergens derived from a wide range of organisms, including fungi, cockroaches and mites, but not pollen or food allergens, induce intracellular maturation and subsequent secretion of IL-33 by epithelial cells,” they continue. “This was a completely unexpected finding because maturation of IL-33 induced by allergens was believed to be an exclusively extra-cellular event mediated by allergen proteases.”
Other Cincinnati Children’s researchers on the study include Mark Rochman, PhD, Yrina Rochman, PhD, Julie Caldwell, PhD, Lydia Mack, MS, Jennifer Felton, PhD, Jeff Habel, PhD, and Alexey Porollo, PhD.
Previous research had established that multiple allergens can induce a similar IL-33 response upon breaching the epithelial layer of mucosal membranes. The Cincinnati Children’s team pinned down the mechanisms at work in the process.
“This breakthrough was made possible by new insights into the role of ripoptosome signaling and caspases in allergic inflammation,” says Brusilovsky.
Specifically, the allergens trigger activity among an interlocked set of cell death-inducing signals called the ripoptosome. This signaling “platform” includes numerous components, but for allergic inflammatory reactions, the key player appears to be a molecular switch called caspase 8. The investigators named the pathway, “Rip-IL-33” because IL-33 is processed (ripped) by the ripoptosome.
Connecting the dots to common allergy response between the ripoptosome and IL-33 was especially important, the researchers say. This is because released IL-33 elicits innate type 2 responses and amplifies responses in both atopic and non-atopic forms of allergic inflammation.
In the last two decades, immunologists have discovered mechanisms by which bacteria and viruses are sensed by the innate immune system, but how allergens are sensed has remained a mystery.
“The discovery of this mechanism is the most important breakthrough in understanding how the innate immune system senses allergens—a question that has puzzled immunologists for a long time,” says Pasare.
Early Progress in Treatment
The Cincinnati Children’s team used its experience at treating a variety of allergies to look for a method to control the rapid-response sensing system. In mouse models they found that inhibiting the activity of caspase 8 reduced the IL-33 response to allergen exposure and limited bronchial inflammation in the lungs.
Importantly, the paper shows that a similar response pathway occurs in humans. “In the human allergic disease eosinophilic esophagitis, we found that ripoptosome activation markers and mature IL-33 levels dynamically correlated with the degree of esophageal eosinophilia and disease activity,” the study states.
In the mouse studies, the most powerful effects were noted when using Q-VD-OPh, a broad-spectrum and irreversible “pan-inhibitor” of caspase 8. However, even though this inhibitor has been used for years in lab studies, it is not considered a safe candidate for human use.
Since Publication
Now, members of the research team are working to further confirm the mechanisms of the RipIL-33 pathway in human allergic reaction. They also are looking for existing drugs, or a new compound, that can safely disrupt the inflammation cycle.
Rothenberg, director of the Division of Allergy and Immunology, has an extensive research portfolio that includes numerous advances in the study and treatment of eosinophilic disorders.
In recognition of his contributions to the field, Rothenberg was honored in October 2022 with election to the National Academy of Medicine.
About the Study
Funding sources for this study included the National Institutes of Health (R37 AI045898, R01 AI123176, R01 AI113125 and R01 CA231303); the Campaign Urging Research for Eosinophilic Disease (CURED); and the Sunshine Charitable Foundation and its supporters, Denise and David Bunning.
Citation
Brusilovsky M, Rochman M, Rochman Y, Caldwell JM, Mack LE, Felton JM, Habel JE, Porollo A, Pasare C, Rothenberg ME. Environmental allergens trigger type 2 inflammation through ripoptosome activation. Nat Immunol. 2021 Oct;22(10):1316-1326.